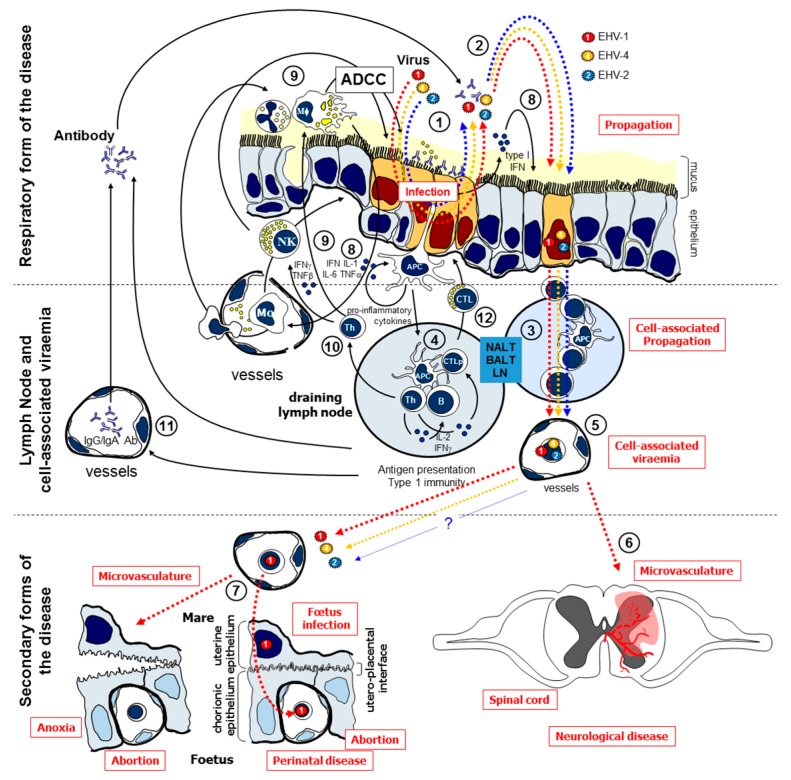
Figure 1.
Equine herpes virus (EHV) infection and immune response, adapted from [12]. EHVs primarily infect the epithelial cells of the upper respiratory tract (1), where they replicate and are excreted (2). EHVs will reach the respiratory lymph nodes (3) where peripheral blood mononuclear cells (PBMC) will be infected (4) and the adaptive immune response will be stimulated. Circulation of infected leucocytes (5) during cell-associated viraemia disseminates EHVs to distant sites such as the central nervous system (EHV-1) (6) or the reproductive tract (EHV-1/4; potentially EHV-2). EHV-infected maternal lymphocytes reach the endometrial capillary during the cell-associated viraemia (7). EHV-1 spreads by cell-to-cell contact to the foetus through the endothelium of endometrial capillaries, the uterine epithelium, the chorionic epithelium and the endothelium of placental capillaries to finally infect foetal lymphocytes. EHV-1 can also infect endometrial endothelial cells inducing uterine pathologies, leading to a premature placenta separation and foetus anoxia. EHV-1 could also reach the central nervous system (6), infect, and damage endothelial cells, potentially leading to microvasculature and equine herpesvirus myeloencephalopathy (EHM). Infection with EHVs also induces the establishment of latency and potential reactivation of infectious virus (not represented here). In terms of immune response, the local synthesis of IFN and IL-6 by infected endothelial cells and innate immune cells will promote antiviral resistance in uninfected cells and up-regulation of major histocompatibility complex (MHC) molecules for viral antigen presentation to the immune system (8). Alveolar macrophages (Mφ) and neutrophils respond to the infection by releasing pro-inflammatory cytokines, leading to the containment of the infection, rise of body temperature, and to the recruitment of further phagocytic and natural killer (NK) cells (9). NK cells are activated by IFN-α and interleukin (IL)-12 which is synthesised by Mφ, and are cytotoxic for virus-infected cells. NK cells synthesise IFN-γ that drives the development of the adaptive immune response. The presentation of viral antigen (10) in lymphoid tissues induces the synthesis of serum (11) or mucosal antibodies able to neutralise excreted virus and to stimulate antibody dependent cell cytotoxicity (ADCC). Virus-specific cytotoxic T lymphocytes (CTL) that lyse infected cells are also stimulated (12). APC: antigen presenting cell; BALT: bronchus-associated lymphoid tissue; LN: lymph node; NALT: nasal-associated lymphoid tissue.