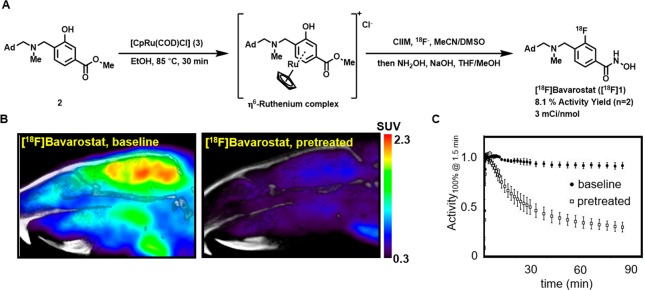
Figure 3.
Rodent PET imaging with [18F]Bavarostat. A: Radiochemical synthesis of [18F]Bavarostat via ruthenium-mediated deoxyfluorination, ClIM = N,N-bis(2,6-diisopropylphenyl)-1-chloroimidazolium chloride. B: Representative sagittal slices summed from 30 to 90 min. C: Averaged (n = 3) time–activity curves of a whole-brain ROI of Sprague–Dawley rats injected with [18F]Bavarostat. In the blocked animals, 1.0 mg·kg–1 unlabeled Bavarostat was injected immediately prior to radiotracer administration, baseline animals treated with vehicle.