Abstract
Obesity and insulin resistance often emerge from positive energy balance and generally are linked to low-grade inflammation. This low-grade inflammation has been called “meta-inflammation” because it is a consequence of the metabolic dysregulation that can accompany overnutrition. One means by which meta-inflammation is linked to insulin resistance is extracellular matrix expansion secondary to meta-inflammation, which we define here as “meta-fibrosis”. The significance of meta-fibrosis is that it reflects a situation in which the extracellular matrix functions as a multi-level integrator of local (for example, mitochondrial reactive oxygen species production) and systemic (for example, inflammation) inputs that couple to cellular processes creating insulin resistance. While adipose tissue extracellular matrix remodeling has received considerable attention, it is becoming increasingly apparent that liver and skeletal muscle extracellular matrix remodeling also contributes to insulin resistance. In this review, we address recent advances in our understanding of energy balance, mitochondrial energetics, meta-inflammation, and meta-fibrosis in the development of insulin resistance.
Keywords: insulin resistance, metabolism, metabolic syndrome, inflammation
Introduction
Advances in industrial and agricultural technology combined with lower rates of energy expenditure through physical activity have had the unintended consequence of creating a dramatic rise in the prevalence of obesity, insulin resistance (IR), hypertension, and dyslipidemia. These comorbidities are principal components of the metabolic syndrome as well as risk factors for type 2 diabetes mellitus and cardiovascular disease. The public health impact of these altered metabolic states is clear when considering that, in 2012, approximately 33% of United States citizens (over 100 million people) were projected to have at least one component of the metabolic syndrome 1.
Positive energy balance at the whole-body level and altered oxidative metabolism at the cellular level are central to the development of IR. However, the conduit linking nutrient status and cellular energetics to pathophysiological states like IR is incompletely defined. In this commentary, we provide a framework for how mitochondrial energetics along with metabolically driven inflammation (meta-inflammation) and extracellular matrix (ECM) remodeling leading to fibrosis (meta-fibrosis) link overnutrition to IR ( Figure 1). As several recent discoveries suggest, there is a great deal to be learned regarding the etiology of IR by studying organ-level physiological events in the context of the extracellular milieu. The focus here will be on metabolism, molecular organization, and cell signaling in the pathogenesis of IR. The important roles of gene transcription and epigenetics in the development of IR are beyond the scope of this commentary. Readers are directed to recent reviews on these topics 2, 3.
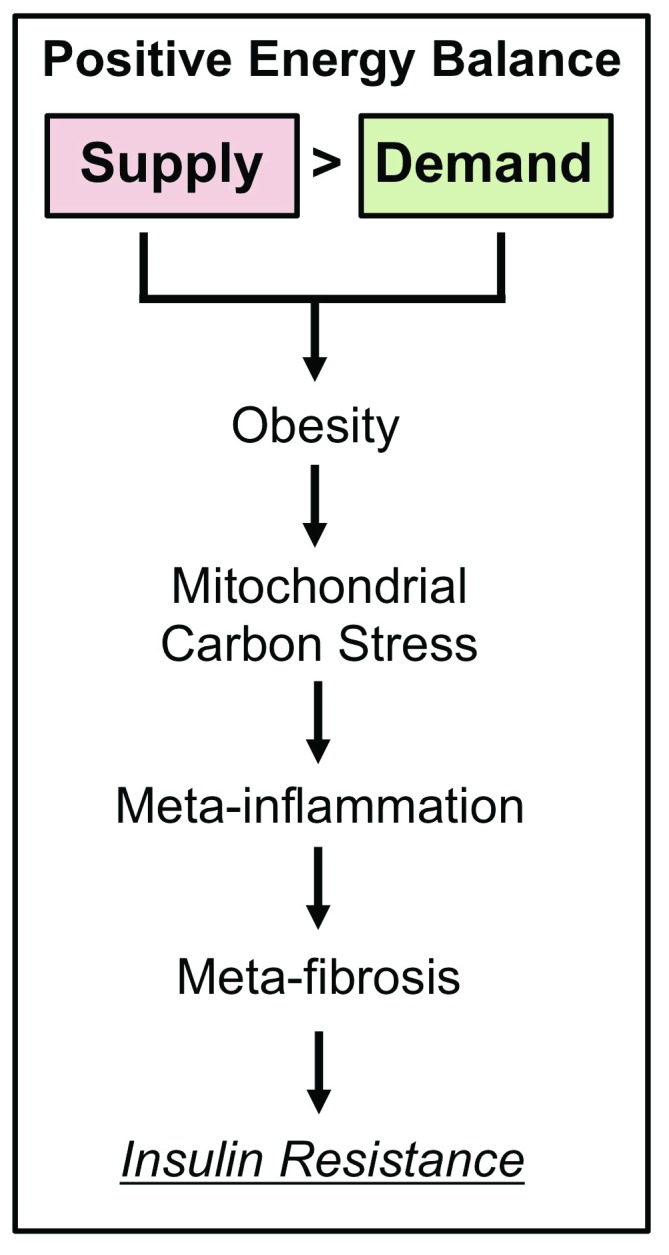
Figure 1. Positive energy balance promotes insulin resistance via metabolism-driven inflammation and fibrosis.
Energy balance is defined as the difference between absorbed dietary macronutrients (Supply) and energy expenditure (Demand). Energy supply is determined by the quantity and composition of macronutrients consumed, whereas energy demand is determined by exercise, non-exercise activity thermogenesis, and resting metabolic rate. A net positive energy balance (Supply > Demand) leads to obesity and a cascade of events that includes mitochondrial carbon stress (that is, an oversupply of macronutrients to mitochondria). This metabolic stress on mitochondria can promote meta-inflammation and meta-fibrosis that ultimately contribute to cellular and systemic insulin resistance.
Energy balance and the metabolic syndrome
Energy balance is defined as the gastrointestinal absorption of dietary macronutrients minus whole-body energy expenditure. Human evolution has selected for traits that facilitate the efficient mobilization, metabolism, and storage of macronutrients. The biological significance of these adaptations lies in the need to store nutrients during times of nutrient excess and the ability to mobilize fuel in situations of nutrient deficiency. Nutrient storage is important for acute bouts of elevated energy expenditure or prolonged periods during which food is not readily available. Indeed, mechanisms for storing excess glucose (glycogen), lipids (triglyceride), and amino acids (protein) obtained from the diet are exquisitely sensitive. While these adaptations have been critical for survival and species propagation, people living in industrialized societies now have easy access to high-calorie foods and do not need to expend considerable energy to obtain their food. This has led to a sustained positive energy balance. Since this is a situation rarely encountered during the course of human evolution, the body is poorly equipped to adapt to dietary excess. As such, the chronic energy surplus incurred by overnutrition and sedentary behavior has become a persistent metabolic burden that leads to adipose tissue expansion and obesity in many individuals 4. Obesity, in turn, is central to the development of IR.
The evolutionarily conserved mechanisms that make survival possible during periods of famine also make humans refractory to weight loss. Resistance to weight loss and weight maintenance is recognized as a primary barrier to improving metabolic health 5. This is most clearly demonstrated when considering the effects of caloric restriction and physical activity on energy balance and body weight. In both obese and non-obese humans 6– 8, prolonged caloric restriction results in significant weight loss, but it is accompanied by reductions in resting metabolic rate (RMR) beyond that which can be accounted for by weight loss alone. Since RMR is a primary contributor to the daily energy budget 9, this represents a significant barrier to long-term weight loss. It is notable that exercise alone is only marginally effective as a therapy for weight loss 10– 12. This is likely due to both metabolic and behavioral obstacles. Exercise training fails to increase RMR in obese individuals with diabetes 13, and this is potentially due to increased metabolic efficiency 14. Exercise training has had mixed results in eliciting weight loss in both rodents 15 and humans 10 and is explained in part by increased food intake. In addition to RMR, “non-exercise activity thermogenesis” (NEAT) is a major contributor to energy expenditure in mice and humans 16. Mice given access to a running wheel increase their physical activity and energy expenditure over a four-week period, but the metabolic cost of activity progressively decreases concurrently with decreased NEAT 17. This is significant because fat gain with overnutrition in humans is positively correlated with an increase in NEAT 18. Whether the bidirectional modulation of NEAT based on whole-body energy balance is modifiable therapeutically remains to be seen but may be a viable strategy for combating obesity. Complicating therapeutic strategies further is a growing body of literature demonstrating that the metabolic adaptations that occur with weight loss predispose an individual to accelerated weight regain and increased adiposity upon cessation of a supervised diet or exercise regimen or both 19. Notably, recent work suggests that glucocorticoid antagonism mitigates the weight regain and IR that occur following cessation of voluntary exercise in rats 20. A better understanding of how humans resist weight loss, even in the setting of obesity, is critically important in that it may reveal novel therapeutic strategies for treating obesity and IR.
Mitochondrial energetics and the pathogenesis of insulin resistance
As the demand-driven terminus of oxidative metabolism, mitochondria are intricately involved in the maintenance of energy balance, and several recent reviews have highlighted the importance of mitochondrial energetics to the etiology of IR 21– 23. At the level of the mitochondrion, energy balance is established by a dynamic rate of carbon flux through the tricarboxylic acid (TCA) cycle that supports ATP production via oxidative phosphorylation. In the setting of overnutrition, there is a supply/demand mismatch that results in excess anaplerotic flux of carbon from fatty acids entering the TCA cycle relative to the ATP demand leading to IR 24. Excessive anaplerotic flux creates a mitochondrial “carbon stress” that has been well documented in both skeletal muscle (SkM) and liver. This carbon stress promotes IR through incompletely defined mechanisms that likely involve post-translational protein modifications that alter insulin signaling or protein trafficking (that is, GLUT4 translocation). The teleological explanation for limiting SkM glucose uptake in the face of excess dietary lipids may be that SkM is unable to efficiently convert excess intracellular glucose to an inert metabolite (that is, fatty acids).
In the liver, greater fatty acid availability accelerates anaplerotic flux contributing to IR that correlates with the severity of non-alcoholic fatty liver disease (NAFLD) in humans 25. This appears to be linked, at least in part, to incomplete β-oxidation in the setting of overnutrition 26. This hypothesis is supported by findings that acyl-carnitine, the carbon chain intermediate of β-oxidation, is increased in human plasma 27 as well as rodent SkM 26. Free carnitine in SkM is also reduced in the setting of obesity or high-fat feeding or both 28, suggesting a reduced capacity to handle excess fatty acids. Collectively, excess dietary fatty acids entering metabolically active tissues overload the mitochondria, leading to IR. A teleological explanation for why mitochondria induce IR may be to mitigate oxidative damage induced by overnutrition 29.
Mitochondria can also engage in cataplerosis, which is removal of carbons from the TCA cycle. In SkM, one proposed role for cataplerosis is as a buffering system to avoid mitochondrial carbon excess that can lead to increased reactive oxygen species (ROS) production during overnutrition 24. SkM cataplerosis occurs in large part via carnitine acetyltransferase (CrAT), an enzyme that is responsible for exporting acetyl and acyl groups bound to carnitine from the mitochondrial matrix into the cytosol. Mice with SkM-specific deletion of CrAT have impaired glucose tolerance and increased oxidative stress 30, illustrating a need for mitochondrial carbon efflux (that is, cataplerosis) to preserve SkM metabolic homeostasis in the setting of overnutrition. In the liver, cataplerosis is essential for the production of both glucose (gluconeogenesis) and ketones (ketogenesis). Predominantly expressed in gluconeogenic organs (liver and kidney), phosphoenolpyruvate carboxykinase (PEPCK) converts oxaloacetate to pyruvate and is a key enzyme for gluconeogenesis. Loss of PEPCK in mice reduces hyperglycemia in leptin receptor–deficient (db/db) diabetic mice 31. Similarly, ketogenesis exerts partial protection against high-fat diet (60% calories from fat)–induced hyperglycemia and fatty liver, primary complications linked to obesity and overnutrition 32. Notably, however, mice fed a ketogenic diet (more than 90% calories from fat) are lean and hypoinsulinemic but also display fatty liver 33, 34. This may be due to the impaired liver mitochondrial respiratory capacity observed in mice fed a short-term (14 days) ketogenic diet 35. Strategies to increase cataplerosis in a tissue- and product-specific fashion could yield valuable strategies for preserving glucose homeostasis and insulin sensitivity but should be considered in the context of also preventing the development of fatty liver.
How does mitochondrial carbon excess promote IR? Carbon turnover that exceeds metabolic demand leads to accumulation of reducing equivalents (NADH and FADH 2) that exert greater “reducing pressure” (that is, more electrons) on the electron transport system 36. This buildup of reducing equivalents in the matrix and electrons within the electron transport system promotes the formation of ROS that modulate a wide variety of normal and pathophysiological cellular processes 37. For example, acute or chronic high-fat feeding increases mitochondrial ROS production that has been shown in some 29, 38– 40, but not all 41, reports to be causal for the development of IR. Notably, fatty acids can also “uncouple” oxidative phosphorylation 42, raising the possibility that mitochondrial oxidative efficiency may be an additional mechanism to manage carbon excess in obesity. Targeting this mechanism may be feasible in light of recent work demonstrating that mitochondrial oxidative efficiency is a dynamic process that is acutely sensitive to energetic demand 43. Historically, the use of mitochondrial uncouplers as therapeutic agents has been met with skepticism following a string of deaths linked to the protonophore 2-dinitrophenol in the 1930s. However, recent efforts have provided new lead compounds that may be promising in the treatment of obesity 44– 46. While mitochondria-targeted therapies are being studied intensively and hold great promise, an alternative approach may be to address downstream effectors of mitochondrial oxidants. The downstream processes affected by mitochondrial oxidants are incompletely defined but include inflammation and expansion of the ECM. The remainder of this article will be spent discussing these processes in the context of their individual, and collective, contributions to the etiology of IR.
Inflammation and extracellular matrix expansion in the etiology of insulin resistance
Low-grade metabolically driven “meta-inflammation” 47 contributes to IR in obesity 48. There are numerous intersecting mechanisms linking inflammation and ROS 49, including a critical role for the innate immune system that is coupled to macrophage infiltration 50, 51. Macrophages recruited with chronic overnutrition are pro-inflammatory (M1; CD11b +) and secrete tumor necrosis factor alpha (TNFα) that has been shown to contribute to IR in adipose, SkM, and liver 52– 54. M1 macrophages also play a critical role in wound healing. It has been observed that the meta-inflammatory response to obesity that includes M1 macrophage infiltration is responsible for the accumulation of ECM proteins in insulin-sensitive tissues 55. The evidence linking these processes in adipose, SkM, and liver is outlined below.
Adipose tissue function is reliant upon, and in certain situations compromised by, the ECM surrounding adipocytes 56. Healthy adipose tissue expansion involves a balance between enzymatic degradation and subsequent synthesis of ECM proteins 57. Pathogenic obesity in humans is characterized by adipose tissue fibrosis due to excessive ECM deposition and reduced ECM degradation that is associated with IR 58– 61. Paradoxically, recent work by Muir et al. 62 showed that diabetics have reduced adipose tissue fibrosis and greater adipose tissue hypertrophy. Genetically obese ( ob/ob) mice have increased expression of genes encoding collagens 63 that is exacerbated by high-fat feeding 64. Genetic loss of the adipose tissue–abundant collagen VI in mice mitigates adipocyte inflammation, diet-induced obesity (DIO), and glucose intolerance while permitting greater adipocyte hypertrophy 63. Beyond collagen, various other ECM components—including osteopontin 65, 66, hyaluronan 67, thrombospondins 68, 69, and microfibril-associated glycoprotein 1 (MAGP1) 70—accumulate in adipose tissue with obesity and contribute to IR. Adipose tissue ECM expansion is attenuated by the anti-diabetic drug metformin 71, a drug that is also known to reduce mitochondrial ROS production 39. Whether metformin improves metabolic health by mitigating mitochondrial ROS production or ECM accumulation or both remains to be addressed directly.
Obesity induces SkM ECM expansion 55, 72, 73 that would be expected to increase the resistance to glucose delivery, an essential controller of glucose uptake 74. Even short-term (28 days) high-fat feeding 75 is sufficient to induce SkM ECM expansion. This appears to be reversible as SkM collagen accumulation is ameliorated in obese mice following exercise training 73 and preventable in mice with genetic enhancement of SkM mitochondrial ROS scavenging 55. A genetic knockout of matrix metalloprotease-9 (MMP-9), a key ECM-degrading enzyme, in obese mice causes increased collagen and a further deterioration of SkM insulin action 76. Treatment with pegylated hyaluronidase causes degradation of hyaluronan and rescues IR in obese mice 77. These studies demonstrate a direct link between ECM accumulation and insulin action in SkM.
In the setting of obesity, circulating lipids are incompletely sequestered in adipose tissue and consequently accumulate in SkM and liver and lead to IR. NAFLD is a primary risk factor for the development of IR and diabetes via liver fibrosis 78, 79. Mice fed a high-fat high-fructose diet exhibit liver fibrosis that accompanies lipid accumulation and IR 80, 81. The extent and scope to which overnutrition alters liver ECM are not completely known, highlighting a need for future studies.
ECM accumulation is recognized as a structural barrier between cells and the vascular space that restricts molecular transport 82. More recently, a body of evidence has emerged indicating that cellular changes that accompany ECM accumulation are receptor-mediated. As such, the ECM is a biomolecular “motherboard” that determines the physical and metabolic properties of the tissue and the cells that they envelope. A greater understanding of how the ECM changes in obesity and the contribution of individual ECM proteins will be necessary in defining extracellular processes impacting metabolic health.
Extracellular matrix expansion and integrins in the setting of obesity
Integrins are a class of receptors that bind ECM proteins and have numerous overlapping functions, including cell adhesion, mechanotransduction, and differentiation 83, 84. ECM receptors are involved in a myriad of receptor signaling events through physical and functional interactions with growth factor receptors, including the insulin receptor 85. In this way, ECM receptors orchestrate dynamic and specific signaling responses to diverse physiological and pathophysiological conditions. Integrins functionally link ECM changes to a multitude of conditions, including IR 86 (summarized in Figure 2).
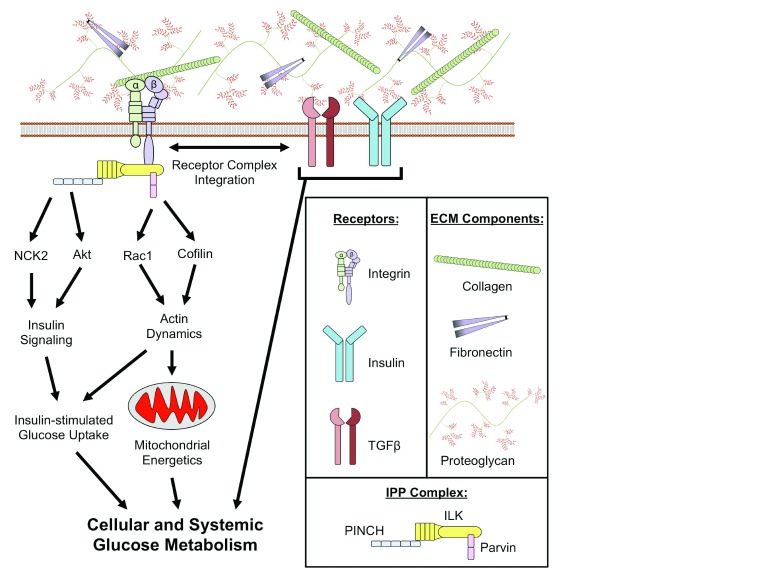
Figure 2. Putative mechanisms for the role of integrins in the development of insulin resistance.
Extracellular matrix (ECM) proteins are ligands for integrins, a family of cell surface receptors. Integrins are linked to the regulation of glucose metabolism through numerous mechanisms. Integrins can co-localize with transforming growth factor beta (TGFβ) and insulin receptors that are key regulators of glucose uptake into tissues. Integrins are also involved in intracellular signaling through the integrin-linked kinase (ILK)/PINCH/Parvin (IPP) complex. PINCH is characterized as a modulator of kinase signaling pathways as it regulates Nck2 and Akt, requisite proteins for insulin signaling. Parvin is involved in the regulation of cytoskeletal dynamics that permit remodeling and translocation of mitochondria and various intracellular proteins (that is, glucose transporters). The integration of integrins with regulatory nodes for glucose metabolism highlights the potential significance of ECM-integrin signaling in the etiology of insulin resistance.
Integrins are heterodimers consisting of α and β subunits with varying ligand specificities and expression in different tissues. Differentiated insulin-sensitive cells from SkM, adipose tissue, and liver express a variety of α subunit isoforms but express only a single β integrin isoform (β1) 87– 89. Whole-body loss of the integrin α1 subunit, a pro-fibrotic integrin receptor subunit that exclusively binds to β1, fails to protect against diet-induced SkM IR in mice; however, loss of the anti-fibrotic α2 isoform that also binds to β1 is protective 55. It is interesting to note that combined SkM and myocardial loss of the integrin β1 subunit results in IR in lean mice 90. Integrin-linked kinase (ILK) is a protein that physically associates with the intracellular tail of the β integrin subunit 90. In contrast to the IR caused by knockout of the integrin β1 subunit in both SkM and myocardium of lean mice 90, SkM-specific loss of ILK (mILK-KO mice) results in improved SkM insulin action in DIO mice 91. Liver-specific deletion of ILK also protects against IR in DIO mice 92. Whether adipocyte ILK deletion has effects on nutrient metabolism remains to be determined.
Despite its name, ILK lacks a functional kinase domain but rather functions as a scaffold for at least 26 high-fidelity binding partners 93. Most notable among these binding proteins are PINCH and parvin, which, together with ILK, form an ILK/PINCH/Parvin (IPP) complex. PINCH consists of two isoforms (PINCH1 and PINCH2) that have both distinct and overlapping cellular functions 94. In the context of glucose homeostasis, PINCH can bind to Nck2, which in turn interacts with insulin receptor substrate-1 (IRS-1) 95, a requisite for insulin signaling. Nck2 is highly expressed in epididymal adipose tissue and its genetic deletion in mice causes IR and increased lipolysis 96. PINCH has also been implicated in the phosphorylation of Akt via interactions with ILK 97. Three ubiquitously expressed isoforms of parvin exist (α, β, and γ). α- and β-parvin both can bind directly to f-actin and in this way regulate cytoskeletal dynamics 98. Parvin-mediated regulation of actin cytoskeletal dynamics is thought to occur, at least in part, via interactions between parvin and the Rho GTPase Rac1 99, 100 and actin depolymerizing factor protein cofilin 101. Rac1 is required for insulin-stimulated glucose uptake and is impaired during IR 102, representing a potential link between integrins and insulin action. A potential role for γ-parvin in the context of insulin action has not been elucidated. Rac1 and cofilin are also involved in the regulation of numerous mitochondrial processes, including fission 103, apoptosis 104, and translocation 105, demonstrating a link between integrins and the regulation of oxidative metabolism. Whether integrins and the IPP complex directly regulate Rac1 or cofilin in obesity is not yet known, nor is it known what role the IPP complex may play in obesity through its other binding partners. A recent report shows that focal adhesion kinase (FAK), an alternative downstream target of integrin activation, can modulate insulin sensitivity through regulation of adipocyte survival 106. In light of the complexities of the ECM, integrins, and intracellular signaling pathways, much remains to be learned about ECM-integrin interactions in IR.
Summary and future directions
The etiology of IR involves both cell-intrinsic regulation of nutrient metabolism and integrated systems pathophysiology. The established paradigm of meta-inflammation coupled with the emerging concept of meta-fibrosis illustrates the complex nature of IR; however, several major questions remain to be addressed. For example, the composition and organization of the ECM must be elucidated so that the contribution of individual proteins or complexes or both can be mechanistically understood. Additionally, the role of downstream intracellular substrates of integrin signaling must be defined in the context of IR and cellular metabolism. The complex nature and broad importance of ECM/integrin function will be better understood through interdisciplinary studies that draw expertise from numerous fields (such as mechanobiology, biophysics, endocrinology, and molecular metabolism). It is anticipated that future studies will provide a more complete understanding of how the ECM functions as a biophysical regulator of whole-body function and support the development of novel therapeutics aimed at treating IR by mitigating meta-fibrosis.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
John Thyfault, Department of Nutrition and Exercise Physiology, University of Missouri, Columbia, Missouri, USA
Jane Shearer, Department of Biochemistry & Molecular Biology, Faculty of Medicine, University of Calgary, Calgary, Alberta T2N1N4, Canada; Faculty of Kinesiology, University of Calgary, Calgary, Alberta T2N1N4, Canada
Michael Riddell, School of Kinesiology and Health Science, York University, Toronto, ON, Canada
Funding Statement
The author(s) declared that this work was funded by National Institutes of Health (grants DK054902, DK050277, DK059637), NIDDK Mouse Metabolic Phenotyping Centers MICROMouse Program (grant 15GRU2558) and American Heart Association (grant 16POST29910001).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
References
- 1. Aguilar M, Bhuket T, Torres S, et al. : Prevalence of the metabolic syndrome in the United States, 2003–2012. JAMA. 2015;313(19):1973–4. 10.1001/jama.2015.4260 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Kang S, Tsai LT, Rosen ED: Nuclear Mechanisms of Insulin Resistance. Trends Cell Biol. 2016;26(5):341–51. 10.1016/j.tcb.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gross B, Pawlak M, Lefebvre P, et al. : PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13(1):36–49. 10.1038/nrendo.2016.135 [DOI] [PubMed] [Google Scholar]
- 4. Endorsed by The Obesity Society, Young DR, Hivert MF, et al. : Sedentary Behavior and Cardiovascular Morbidity and Mortality: A Science Advisory From the American Heart Association. Circulation. 2016;134(13):e262–79. 10.1161/CIR.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 5. Donnelly JE, Blair SN, Jakicic JM, et al. : American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. 10.1249/MSS.0b013e3181949333 [DOI] [PubMed] [Google Scholar]
- 6. Tremblay A, Chaput JP: Adaptive reduction in thermogenesis and resistance to lose fat in obese men. Br J Nutr. 2009;102(4):488–92. 10.1017/S0007114508207245 [DOI] [PubMed] [Google Scholar]
- 7. Doucet E, St-Pierre S, Alméras N, et al. : Evidence for the existence of adaptive thermogenesis during weight loss. Br J Nutr. 2001;85(6):715–23. 10.1079/BJN2001348 [DOI] [PubMed] [Google Scholar]
- 8. Redman LM, Heilbronn LK, Martin CK, et al. : Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One. 2009;4(2):e4377. 10.1371/journal.pone.0004377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Speakman JR, Selman C: Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62(3):621–34. 10.1079/PNS2003282 [DOI] [PubMed] [Google Scholar]
- 10. Thomas DM, Bouchard C, Church T, et al. : Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes Rev. 2012;13(10):835–47. 10.1111/j.1467-789X.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Byrne NM, Wood RE, Schutz Y, et al. : Does metabolic compensation explain the majority of less-than-expected weight loss in obese adults during a short-term severe diet and exercise intervention? Int J Obes (Lond). 2012;36(11):1472–8. 10.1038/ijo.2012.109 [DOI] [PubMed] [Google Scholar]
- 12. Shaw K, Gennat H, O'Rourke P, et al. : Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006; (4):CD003817. 10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jennings AE, Alberga A, Sigal RJ, et al. : The effect of exercise training on resting metabolic rate in type 2 diabetes mellitus. Med Sci Sports Exerc. 2009;41(8):1558–65. 10.1249/MSS.0b013e31819d6a6f [DOI] [PubMed] [Google Scholar]
- 14. Amati F, Dubé JJ, Shay C, et al. : Separate and combined effects of exercise training and weight loss on exercise efficiency and substrate oxidation. J Appl Physiol (1985). 2008;105(3):825–31. 10.1152/japplphysiol.90384.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maclean PS, Bergouignan A, Cornier MA, et al. : Biology's response to dieting: the impetus for weight regain. Am J Physiol Regul Integr Comp Physiol. 2011;301(3):R581–600. 10.1152/ajpregu.00755.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garland T, Jr, Schutz H, Chappell MA, et al. : The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214(Pt 2):206–29. 10.1242/jeb.048397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Neal TJ, Friend DM, Guo J, et al. : Increases in Physical Activity Result in Diminishing Increments in Daily Energy Expenditure in Mice. Curr Biol. 2017;27(3):423–30. 10.1016/j.cub.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Levine JA, Eberhardt NL, Jensen MD: Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–4. 10.1126/science.283.5399.212 [DOI] [PubMed] [Google Scholar]
- 19. MacLean PS, Higgins JA, Giles ED, et al. : The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16 Suppl 1:45–54. 10.1111/obr.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Teich T, Dunford EC, Porras DP, et al. : Glucocorticoid antagonism limits adiposity rebound and glucose intolerance in young male rats following the cessation of daily exercise and caloric restriction. Am J Physiol Endocrinol Metab. 2016;311(1):E56–68. 10.1152/ajpendo.00490.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Lark DS, Fisher-Wellman KH, Neufer PD: High-fat load: mechanism(s) of insulin resistance in skeletal muscle. Int J Obes Suppl. 2012;2(Suppl 2):S31–S36. 10.1038/ijosup.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hesselink MK, Schrauwen-Hinderling V, Schrauwen P: Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(11):633–45. 10.1038/nrendo.2016.104 [DOI] [PubMed] [Google Scholar]
- 23. Theurey P, Rieusset J: Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol Metab. 2017;28(1):32–45. 10.1016/j.tem.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 24. Muoio DM, Neufer PD: Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metab. 2012;15(5):595–605. 10.1016/j.cmet.2012.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Satapati S, Kucejova B, Duarte JA, et al. : Mitochondrial metabolism mediates oxidative stress and inflammation in fatty liver. J Clin Invest. 2015;125(12):4447–62. 10.1172/JCI82204 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Koves TR, Ussher JR, Noland RC, et al. : Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45–56. 10.1016/j.cmet.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 27. Adams SH, Hoppel CL, Lok KH, et al. : Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139(6):1073–81. 10.3945/jn.108.103754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noland RC, Koves TR, Seiler SE, et al. : Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem. 2009;284(34):22840–52. 10.1074/jbc.M109.032888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hoehn KL, Salmon AB, Hohnen-Behrens C, et al. : Insulin resistance is a cellular antioxidant defense mechanism. Proc Natl Acad Sci U S A. 2009;106(42):17787–92. 10.1073/pnas.0902380106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Muoio DM, Noland RC, Kovalik JP, et al. : Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012;15(5):764–77. 10.1016/j.cmet.2012.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gómez-Valadés AG, Méndez-Lucas A, Vidal-Alabró A, et al. : Pck1 gene silencing in the liver improves glycemia control, insulin sensitivity, and dyslipidemia in db/db mice. Diabetes. 2008;57(8):2199–210. 10.2337/db07-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cotter DG, Ercal B, Huang X, et al. : Ketogenesis prevents diet-induced fatty liver injury and hyperglycemia. J Clin Invest. 2014;124(12):5175–90. 10.1172/JCI76388 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Garbow JR, Doherty JM, Schugar RC, et al. : Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300(6):G956–67. 10.1152/ajpgi.00539.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Klein MS, Newell C, Bomhof MR, et al. : Metabolomic Modeling To Monitor Host Responsiveness to Gut Microbiota Manipulation in the BTBR T+tf/j Mouse. J Proteome Res. 2016;15(4):1143–50. 10.1021/acs.jproteome.5b01025 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Newell C, Shutt TE, Ahn Y, et al. : Tissue Specific Impacts of a Ketogenic Diet on Mitochondrial Dynamics in the BTBR T+tf/j Mouse. Front Physiol. 2016;7:654. 10.3389/fphys.2016.00654 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Fisher-Wellman KH, Neufer PD: Linking mitochondrial bioenergetics to insulin resistance via redox biology. Trends Endocrinol Metab. 2012;23(3):142–53. 10.1016/j.tem.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jones DP: Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295(4):C849–68. 10.1152/ajpcell.00283.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson EJ, Lustig ME, Boyle KE, et al. : Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–81. 10.1172/JCI37048 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Kane DA, Anderson EJ, Price JW, 3rd, et al. : Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med. 2010;49(6):1082–7. 10.1016/j.freeradbiomed.2010.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lark DS, Kang L, Lustig ME, et al. : Enhanced mitochondrial superoxide scavenging does not improve muscle insulin action in the high fat-fed mouse. PLoS One. 2015;10(5):e0126732. 10.1371/journal.pone.0126732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paglialunga S, van Bree B, Bosma M, et al. : Targeting of mitochondrial reactive oxygen species production does not avert lipid-induced insulin resistance in muscle tissue from mice. Diabetologia. 2012;55(10):2759–68. 10.1007/s00125-012-2626-x [DOI] [PubMed] [Google Scholar]
- 42. Anderson EJ, Yamazaki H, Neufer PD: Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282(43):31257–66. 10.1074/jbc.M706129200 [DOI] [PubMed] [Google Scholar]
- 43. Lark DS, Torres MJ, Lin CT, et al. : Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers. Am J Physiol Cell Physiol. 2016;311(2):C239–45. 10.1152/ajpcell.00124.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kenwood BM, Weaver JL, Bajwa A, et al. : Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metab. 2014;3(2):114–23. 10.1016/j.molmet.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Ost M, Keipert S, Klaus S: Targeted mitochondrial uncoupling beyond UCP1 - The fine line between death and metabolic health. Biochimie. 2017;134:77–85. 10.1016/j.biochi.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 46. Lou P, Hansen BS, Olsen PH, et al. : Mitochondrial uncouplers with an extraordinary dynamic range. Biochem J. 2007;407(1):129–40. 10.1042/BJ20070606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hotamisligil GS: Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 48. Xu H, Barnes GT, Yang Q, et al. : Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–30. 10.1172/JCI19451 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Mittal M, Siddiqui MR, Tran K, et al. : Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–67. 10.1089/ars.2012.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Man K, Kutyavin VI, Chawla A: Tissue Immunometabolism: Development, Physiology, and Pathobiology. Cell Metab. 2017;25(1):11–26. 10.1016/j.cmet.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lackey DE, Olefsky JM: Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12(1):15–28. 10.1038/nrendo.2015.189 [DOI] [PubMed] [Google Scholar]
- 52. Hotamisligil GS, Shargill NS, Spiegelman BM: Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. 10.1126/science.7678183 [DOI] [PubMed] [Google Scholar]
- 53. Plomgaard P, Bouzakri K, Krogh-Madsen R, et al. : Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54(10):2939–45. 10.2337/diabetes.54.10.2939 [DOI] [PubMed] [Google Scholar]
- 54. Lang CH, Dobrescu C, Bagby GJ: Tumor necrosis factor impairs insulin action on peripheral glucose disposal and hepatic glucose output. Endocrinology. 1992;130(1):43–52. 10.1210/endo.130.1.1727716 [DOI] [PubMed] [Google Scholar]
- 55. Kang L, Ayala JE, Lee-Young RS, et al. : Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60(2):416–26. 10.2337/db10-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rutkowski JM, Stern JH, Scherer PE: The cell biology of fat expansion. J Cell Biol. 2015;208(5):501–12. 10.1083/jcb.201409063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Crewe C, An YA, Scherer PE: The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74–82. 10.1172/JCI88883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vila IK, Badin PM, Marques MA, et al. : Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Rep. 2014;7(4):1116–29. 10.1016/j.celrep.2014.03.062 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Spencer M, Yao-Borengasser A, Unal R, et al. : Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299(6):E1016–27. 10.1152/ajpendo.00329.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dankel SN, Svärd J, Matthä S, et al. : COL6A3 expression in adipocytes associates with insulin resistance and depends on PPARγ and adipocyte size. Obesity (Silver Spring). 2014;22(8):1807–13. 10.1002/oby.20758 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 61. Guglielmi V, Cardellini M, Cinti F, et al. : Omental adipose tissue fibrosis and insulin resistance in severe obesity. Nutr Diabetes. 2015;5:e175. 10.1038/nutd.2015.22 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Muir LA, Neeley CK, Meyer KA, et al. : Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity (Silver Spring). 2016;24(3):597–605. 10.1002/oby.21377 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Khan T, Muise ES, Iyengar P, et al. : Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29(6):1575–91. 10.1128/MCB.01300-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huber J, Löffler M, Bilban M, et al. : Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond). 2007;31(6):1004–13. 10.1038/sj.ijo.0803511 [DOI] [PubMed] [Google Scholar]
- 65. Kiefer FW, Zeyda M, Todoric J, et al. : Osteopontin expression in human and murine obesity: extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149(3):1350–7. 10.1210/en.2007-1312 [DOI] [PubMed] [Google Scholar]
- 66. Nomiyama T, Perez-Tilve D, Ogawa D, et al. : Osteopontin mediates obesity-induced adipose tissue macrophage infiltration and insulin resistance in mice. J Clin Invest. 2007;117(10):2877–88. 10.1172/JCI31986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Han CY, Subramanian S, Chan CK, et al. : Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56(9):2260–73. 10.2337/db07-0218 [DOI] [PubMed] [Google Scholar]
- 68. Varma V, Yao-Borengasser A, Bodles AM, et al. : Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57(2):432–9. 10.2337/db07-0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Inoue M, Jiang Y, Barnes RH, 2nd, et al. : Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology. 2013;154(12):4548–59. 10.1210/en.2013-1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Craft CS, Pietka TA, Schappe T, et al. : The extracellular matrix protein MAGP1 supports thermogenesis and protects against obesity and diabetes through regulation of TGF-β. Diabetes. 2014;63(6):1920–32. 10.2337/db13-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Luo T, Nocon A, Fry J, et al. : AMPK Activation by Metformin Suppresses Abnormal Extracellular Matrix Remodeling in Adipose Tissue and Ameliorates Insulin Resistance in Obesity. Diabetes. 2016;65(8):2295–310. 10.2337/db15-1122 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Berria R, Wang L, Richardson DK, et al. : Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab. 2006;290(3):E560–5. 10.1152/ajpendo.00202.2005 [DOI] [PubMed] [Google Scholar]
- 73. Pincu Y, Linden MA, Zou K, et al. : The effects of high fat diet and moderate exercise on TGFβ1 and collagen deposition in mouse skeletal muscle. Cytokine. 2015;73(1):23–9. 10.1016/j.cyto.2015.01.013 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Wasserman DH: Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296(1):E11–21. 10.1152/ajpendo.90563.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tam CS, Chaudhuri R, Hutchison AT, et al. : Skeletal muscle extracellular matrix remodeling after short-term overfeeding in healthy humans. Metab Clin Exp. 2017;67:26–30. 10.1016/j.metabol.2016.10.009 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Kang L, Mayes WH, James FD, et al. : Matrix metalloproteinase 9 opposes diet-induced muscle insulin resistance in mice. Diabetologia. 2014;57(3):603–13. 10.1007/s00125-013-3128-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kang L, Lantier L, Kennedy A, et al. : Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes. 2013;62(6):1888–96. 10.2337/db12-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Ekstedt M, Franzén LE, Mathiesen UL, et al. : Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. 10.1002/hep.21327 [DOI] [PubMed] [Google Scholar]
- 79. McCullough AJ: The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8(3):521–33, viii. 10.1016/j.cld.2004.04.004 [DOI] [PubMed] [Google Scholar]
- 80. Kohli R, Kirby M, Xanthakos SA, et al. : High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology. 2010;52(3):934–44. 10.1002/hep.23797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Luo Y, Burrington CM, Graff EC, et al. : Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am J Physiol Endocrinol Metab. 2016;310(6):E418–39. 10.1152/ajpendo.00319.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Sun K, Tordjman J, Clément K, et al. : Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–7. 10.1016/j.cmet.2013.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gattazzo F, Urciuolo A, Bonaldo P: Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–19. 10.1016/j.bbagen.2014.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ringer P, Colo G, Fässler R, et al. : Sensing the mechano-chemical properties of the extracellular matrix. Matrix Biol. 2017; pii: S0945-053X(17)30018-5. 10.1016/j.matbio.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 85. Kim SH, Turnbull J, Guimond S: Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–51. 10.1530/JOE-10-0377 [DOI] [PubMed] [Google Scholar]
- 86. Williams AS, Kang L, Wasserman DH: The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26(7):357–66. 10.1016/j.tem.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schwander M, Shirasaki R, Pfaff SL, et al. : Beta1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J Neurosci. 2004;24(37):8181–91. 10.1523/JNEUROSCI.1345-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schwander M, Leu M, Stumm M, et al. : Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4(5):673–85. 10.1016/S1534-5807(03)00118-7 [DOI] [PubMed] [Google Scholar]
- 89. Liadaki K, Casar JC, Wessen M, et al. : β4 integrin marks interstitial myogenic progenitor cells in adult murine skeletal muscle. J Histochem Cytochem. 2012;60(1):31–44. 10.1369/0022155411428991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zong H, Bastie CC, Xu J, et al. : Insulin resistance in striated muscle-specific integrin receptor beta1-deficient mice. J Biol Chem. 2009;284(7):4679–88. 10.1074/jbc.M807408200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kang L, Mokshagundam S, Reuter B, et al. : Integrin-Linked Kinase in Muscle Is Necessary for the Development of Insulin Resistance in Diet-Induced Obese Mice. Diabetes. 2016;65(6):1590–600. 10.2337/db15-1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Williams AS, Trefts E, Lantier L, et al. : Integrin-Linked Kinase Is Necessary for the Development of Diet-Induced Hepatic Insulin Resistance. Diabetes. 2017;66(2):325–34. 10.2337/db16-0484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dobreva I, Fielding A, Foster LJ, et al. : Mapping the integrin-linked kinase interactome using SILAC. J Proteome Res. 2008;7(4):1740–9. 10.1021/pr700852r [DOI] [PubMed] [Google Scholar]
- 94. Kovalevich J, Tracy B, Langford D: PINCH: More than just an adaptor protein in cellular response. J Cell Physiol. 2011;226(4):940–7. 10.1002/jcp.22437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Tu Y, Liang L, Frank SJ, et al. : Src homology 3 domain-dependent interaction of Nck-2 with insulin receptor substrate-1. Biochem J. 2001;354(Pt 2):315–22. 10.1042/bj3540315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dusseault J, Li B, Haider N, et al. : Nck2 Deficiency in Mice Results in Increased Adiposity Associated With Adipocyte Hypertrophy and Enhanced Adipogenesis. Diabetes. 2016;65(9):2652–66. 10.2337/db15-1559 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Xu Z, Fukuda T, Li Y, et al. : Molecular dissection of PINCH-1 reveals a mechanism of coupling and uncoupling of cell shape modulation and survival. J Biol Chem. 2005;280(30):27631–7. 10.1074/jbc.M504189200 [DOI] [PubMed] [Google Scholar]
- 98. Nikolopoulos SN, Turner CE: Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol. 2000;151(7):1435–48. 10.1083/jcb.151.7.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Berrier AL, Martinez R, Bokoch GM, et al. : The integrin beta tail is required and sufficient to regulate adhesion signaling to Rac1. J Cell Sci. 2002;115(Pt 22):4285–91. 10.1242/jcs.00109 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Pignatelli J, LaLonde SE, LaLonde DP, et al. : Actopaxin (α-parvin) phosphorylation is required for matrix degradation and cancer cell invasion. J Biol Chem. 2012;287(44):37309–20. 10.1074/jbc.M112.385229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shibue T, Brooks MW, Weinberg RA: An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell. 2013;24(4):481–98. 10.1016/j.ccr.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 102. Sylow L, Jensen TE, Kleinert M, et al. : Rac1 signaling is required for insulin-stimulated glucose uptake and is dysregulated in insulin-resistant murine and human skeletal muscle. Diabetes. 2013;62(6):1865–75. 10.2337/db12-1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Li S, Xu S, Roelofs BA, et al. : Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission. J Cell Biol. 2015;208(1):109–23. 10.1083/jcb.201404050 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 104. Chua BT, Volbracht C, Tan KO, et al. : Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003;5(12):1083–9. 10.1038/ncb1070 [DOI] [PubMed] [Google Scholar]
- 105. Matveeva EA, Venkova LS, Chernoivanenko IS, et al. : Vimentin is involved in regulation of mitochondrial motility and membrane potential by Rac1. Biol Open. 2015;4(10):1290–7. 10.1242/bio.011874 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 106. Luk CT, Shi SY, Cai EP, et al. : FAK signalling controls insulin sensitivity through regulation of adipocyte survival. Nat Commun. 2017;8:14360. 10.1038/ncomms14360 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation