Figure 3.
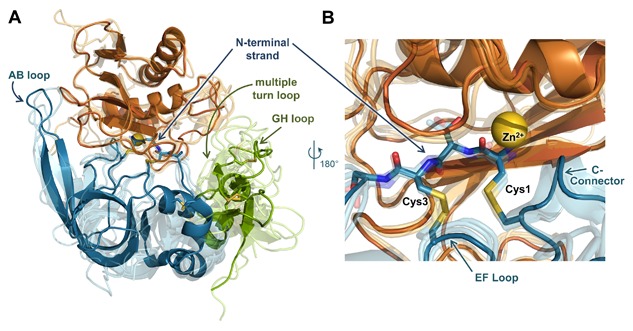
MMP/TIMP interfaces feature a conserved core interaction and diverse peripheral interactions. (A) The structure of the representative complex of MMP‐10CAT (orange) with TIMP‐2 (N‐terminal domain blue, C‐terminal domain green) (PDB ID: 4ILW) [Batra et al., 2013] is shown; the catalytic zinc ion is rendered as a yellow sphere. Four additional MMPCAT/TIMP structures (semi‐transparent) are superposed to highlight regions of structural conservation versus diversity: MMP‐10CAT/TIMP‐1 (PDB ID: 3V96) [Batra et al., 2012a], MMP‐3CAT/TIMP‐1 (PDB ID: 1UEA)[Gomis‐Ruth et al., 1997], MMP‐14CAT/TIMP‐2 (PDB ID: 1BQQ)[Fernandez‐Catalan et al., 1998], and MMP‐13CAT/TIMP‐2 (PDB ID: 2E2D)[Maskos et al., 2007]. Diverse interactions with different MMPs are formed by peripheral TIMP epitopes of the AB, GH, and multiple turn loops. (B) Closer view of the superposed complexes reveals conserved positioning and interactions of the TIMP core epitope, including N‐terminal residues 1–4, the EF loop, and C‐connector loop. The amine terminus of residue Cys‐1 coordinates directly to the catalytic zinc ion. Figure was generated using PyMOL (Schrodinger, LLC).
