Figure 1. Inter-segment RNA-RNA interactions in rotaviruses examined by FCCS.
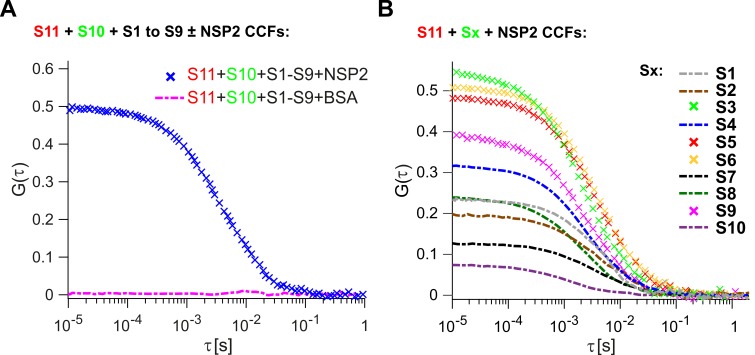
(A) Interactions between fluorescently labelled ssRNAs S10 and S11, incubated in the presence of an equimolar set of unlabelled ssRNAs S1 to S9, were probed by FCCS, as described in Materials and methods. A full set of S1-S11 ssRNAs does not associate into a higher order RNA complex (cross-correlation function amplitude, CCF ≈ 0, shown as a dashed magenta line). In contrast, incubation of the mixture of all eleven genomic ssRNAs in the presence of NSP2 results in a strong interaction between S10 and S11 (high amplitude of CCF shown in blue). (B) Pairwise inter-segment RNA-RNA interactions in RVs. Fluorescently labelled ssRNA S11 (red), incubated with RNA Sx (where x – any other genomic ssRNA S1-S10) in the presence of NSP2, as described in Materials and methods. CCF amplitudes were normalised by their respective auto-correlation functions (ACFs), reflecting the differences in the affinities between S11 and other ssRNAs, with the strongest interactions detected for the RNA-RNA combinations S11:S3, S11:S5 and S11:S6.
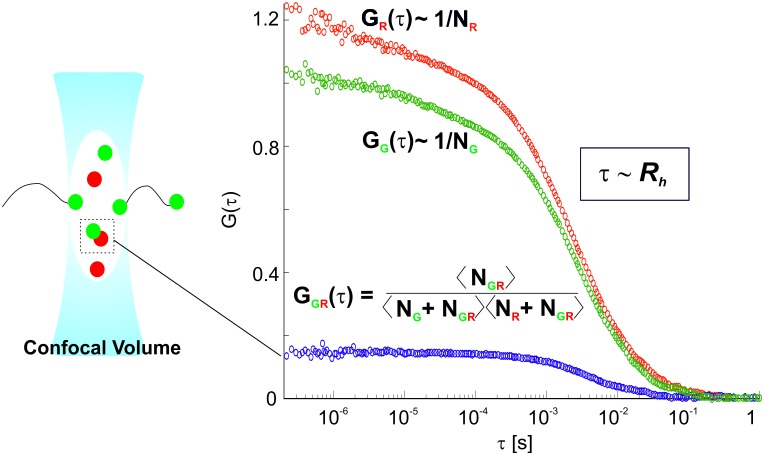
Figure 1—figure supplement 1. Principles of Dual-color Fluorescence Cross-Correlation Spectroscopy (FCCS).
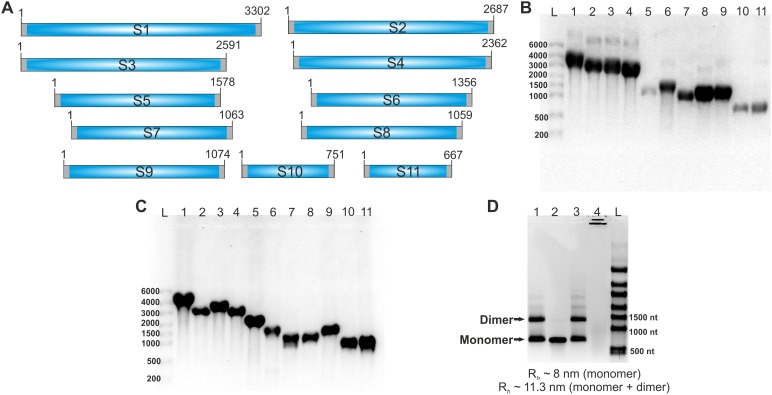
Figure 1—figure supplement 2. RV genomic segment ssRNAs used in the study.
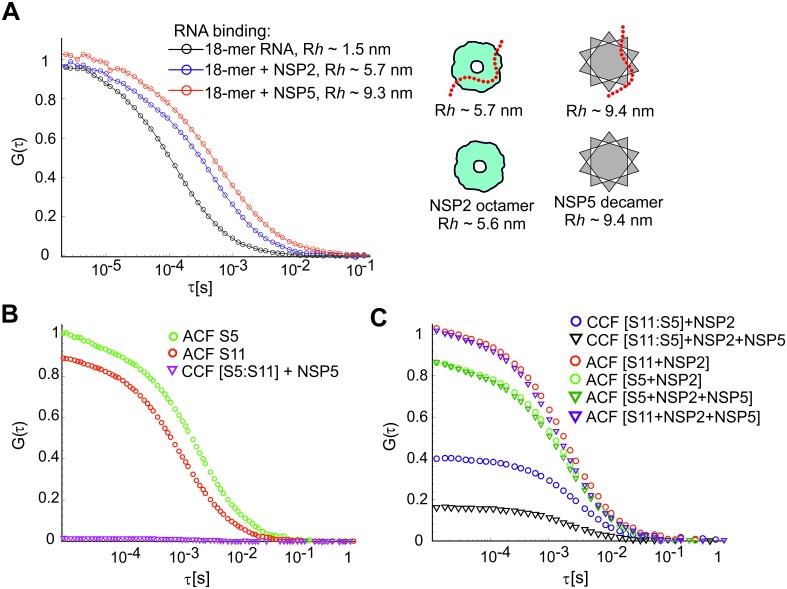
Figure 1—figure supplement 3. RNA-RNA interactions examined by FCCS in the presence of NSP2 and NSP5.
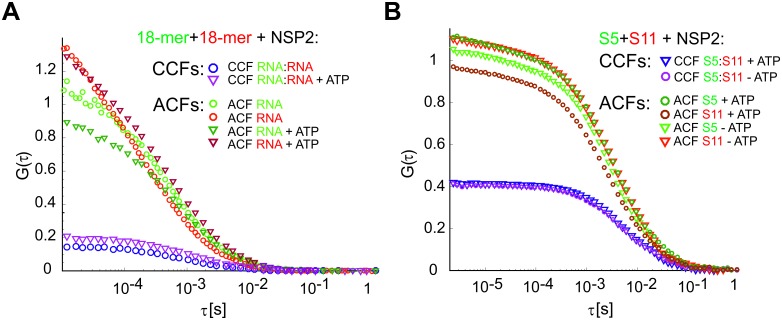
Figure 1—figure supplement 4. Effect of ATP on the RNA-binding properties of NSP2.
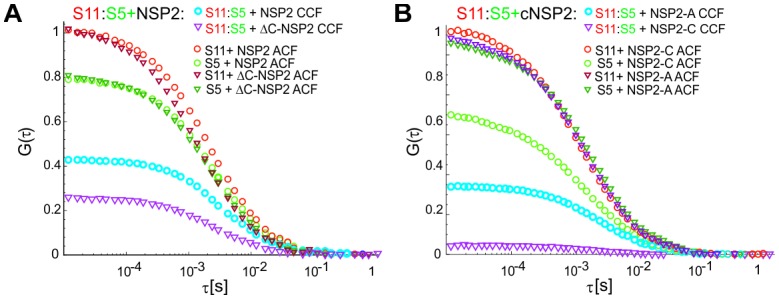
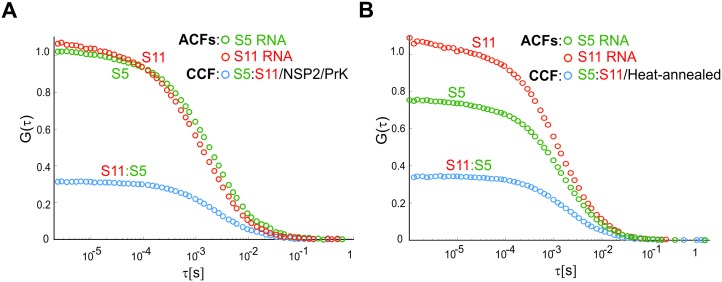
Figure 1—figure supplement 5. S5:S11 RNA-RNA interactions examined in the presence of RV group A NSP2 mutant ΔC-NSP2 and RV group C NSP2.