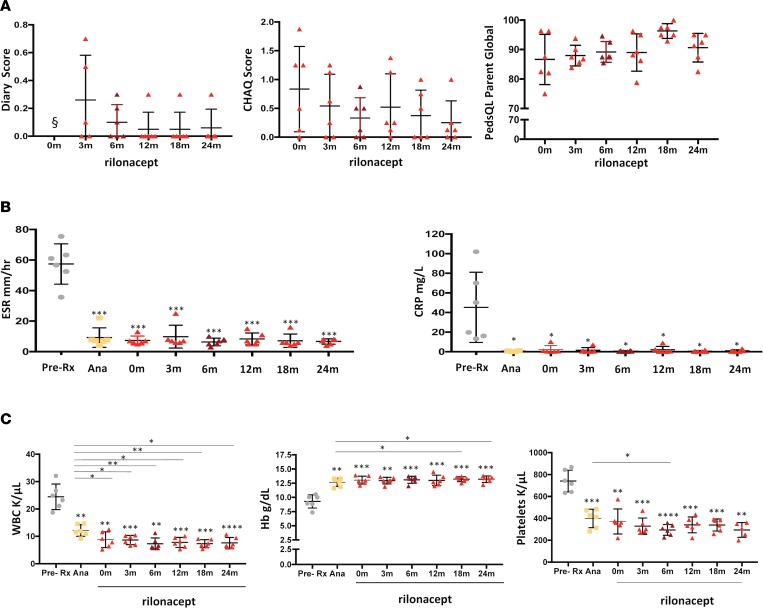
Figure 2. Clinical and laboratory responses in patients with deficiency of IL-1 receptor antagonist treated with rilonacept.
(A) Diary scores, Childhood Health Assessment Questionnaires (CHAQ) scores, and Pediatric Quality of Life (PedsQL) assessments were obtained at the baseline visit and after 3, 6, 12, 18, and 24 months on rilonacept (n = 6). § denotes that the diary score was 0 at baseline. The diary, CHAQ, and PedsQL scores did not significantly change from baseline. The transient elevation on the diary score at 3 months reflects the presence of micropustules in keratinized skin areas (i.e., elbow and knees). (B) C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR) were compared between the pretreatment period, the anakinra treatment period, and the visits after initiation of rilonacept (baseline visit and after 3, 6, 12, 18, and 24 months). (C) White blood cells count (WBC), hemoglobin (Hb), and platelet count changes pretreatment, on anakinra, and on the respective rilonacept visits (baseline and after 3, 6, 12, 18, and 24 months) were compared. Comparisons among pretreatment, on anakinra, and respective rilonacept visits were made using paired t tests (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001). Dots represent individual patients, and lines show the mean ± SD. Asterisks over values at test points indicate comparisons between pretreatment and anakinra as well as pretreatment and rilonacept. Asterisks over lines indicate comparisons between anakinra versus rilonacept.