Figure 2. Cdc37 architecture and interactions with Cdk4 kinase in context of Hsp90.
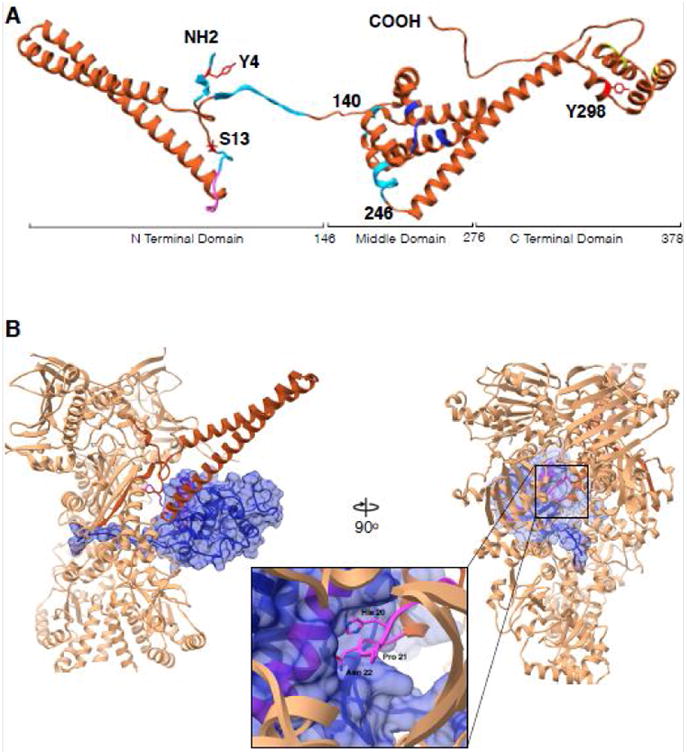
(A) Cdc37 structure synthesized from different studies. Residues 1-260 are from cryoEM structure (PDB:5FWL), 261-293 from Hsp90-Cdc37 domain crystal structure (PDB:1US7) and 294-378 from the ensemble solution NMR structure (PDB:2N5X). Cyan color indicates regions interacting with Hsp90 in Hsp90-Cdc37-Cdk4 complex, pink is the kinase interacting loop (HPN motif), blue color are Hsp90 interacting residues as in the crystal structure and in yellow are residues implicated in additional interactions with bRaf kinase by NMR. Y4, S13 and Y298 are known to be phosphorylated and are marked in red. (B) Interactions between Cdc37 and Cdk4 in the context of Hsp90. Only the Cdc37 N terminal domain is depicted for clarity (piper) interacting with the Cdk4 αE helix (pink helix within blue surface) with the loop harboring HPN motif on Cdc37 (pink, zoomed in). Kinase (omitting residues 1-85) depicted as blue transparent surface, threads β4- β5 strands through the lumen of Hsp90 with Hsp90 clamping around them at its middle domain-C terminal domain junction.
