Figure 4. Hsp90-kinase cycle.
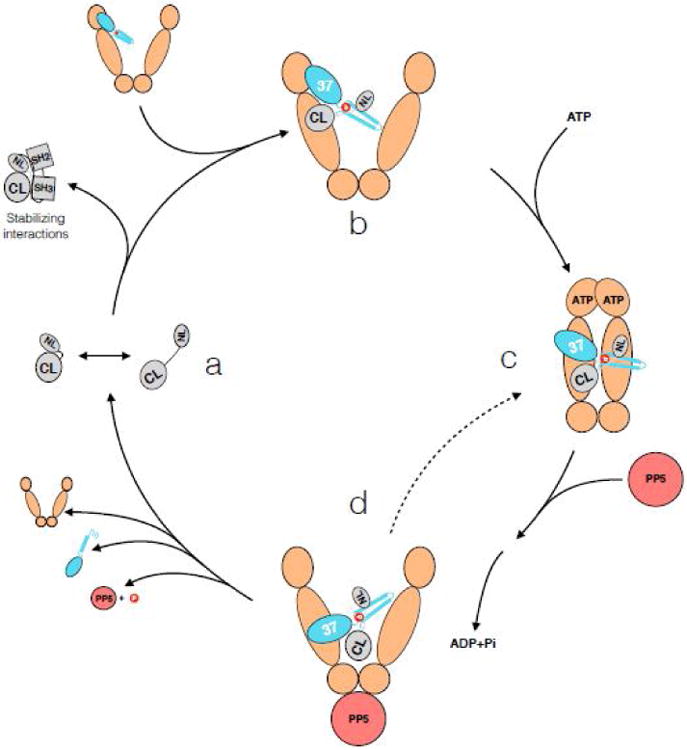
Kinases sample partially open states (CL- C-lobe, NL – N-lobe) (state a). If the kinase spends too much time open at the lobes, for example during early folded state or with a constitutive client, Cdc37 wedges in between the two kinase lobes with its N terminal domain, further unfolding the kinase (state b). Based on NMR data, the C terminus of Cdc37 also makes some kinase interactions at this point. Cdc37 interacts with the N terminal domain of an open Hsp90, utilizing the contacts as in the crystal structure (modeled in Figure 3). Upon binding ATP, Hsp90 undergoes closure, clamping around the unfolded kinase β4-β5 strands, and Cdc37 migrates to the middle domain (state c). Upon ATP hydrolysis by Hsp90 (potentially sequential, with an asymmetric state in between), Hsp90 opens, giving a chance for the kinase to fold (state d). If this was an initial folding interaction for a “non client”, or there is a stabilizing binding partner present nearby for the constitutive client, the stabilized and folded N lobe outcompetes Cdc37 for the C lobe, dissociating the kinase from the complex. However, if there are no stabilizing interactions, Hsp90 may re-bind ATP and enter another cycle (dashed line). This way, the kinase is safely held in a partially folded inactive state, always ready to interact with an appropriate binding partner, checking for its presence with the frequency of Hsp90's ATPase. Dynamic phosphorylation and dephosphorylation by Yes and CKII kinases, and PP5 phosphatase, add a layer of regulation.
