Figure 5. Imagined kinase folding free energy landscape.
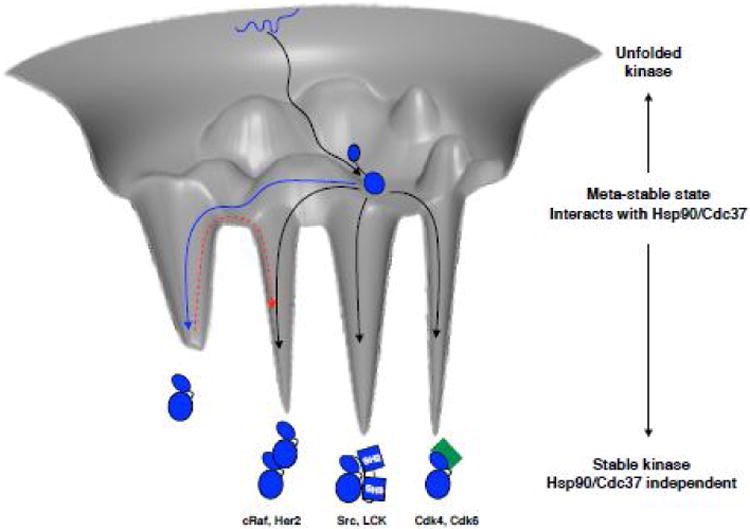
Kinase starts out in an unfolded state (depicted as blue squiggly line at the top of the folding funnel), and likely with the help of general chaperone machinery reaches a partially folded state, perhaps with a rather well folded C-lobe (black arrow to the middle of the folding funnel). From this ensemble of metastable states the kinase interacts with Hsp90-Cdc37. Non-client kinases would then readily access a distinct, well-folded native state and quickly escape the metastable ensemble (blue arrow). If the kinase is a constitutive client, it spends most of its time in the ensemble of partially folded states, without access to a stable state by itself and needs an interacting partner to be stabilized, be it cyclin (green), SH2-SH3 domains or even other kinases (black arrows into deep energy wells). Examples of client kinases representing each mode of stabilization are given below the cartoons. Non-client kinases would have to pay a considerable energy penalty to transition out of the energetically deep native state into an effector bound state (red dashed arrow). Hsp90 client kinases however, being in a structurally uncommitted state are always ready to interact with effectors, foregoing the need to pay the energy penalty. Importantly, Hsp90-Cdc37 may also catalyze the transitions between interactions with different effectors.
