Figure I. Hsp90's ATPase cycle.
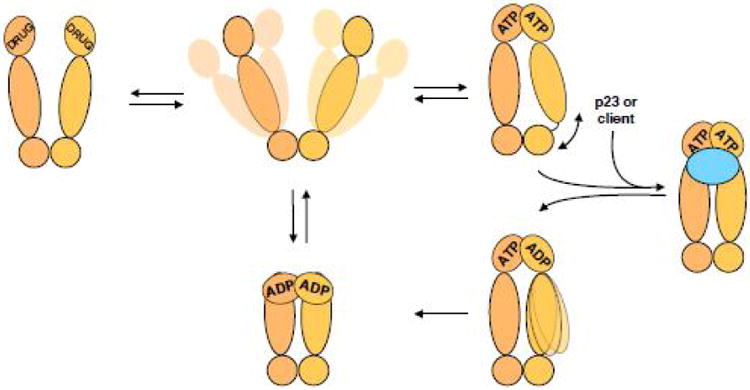
Hsp90 dimer (shades of tan) samples a variety of open states without a nucleotide. Upon binding of ATP, Hsp90 adopts a closed state with the N terminal domains rotating and dimerizing. Without additional binding partners such closed state is asymmetric at the interface between Hsp90's middle and C terminal domains. However, binding of co-chaperones like p23 or co-chaperones with clients, like Cdc37-kinase (either case is shown as blue oval) causes Hsp90 to adopt a symmetric state. ATP is then hydrolyzed in the N terminal domains, potentially sequentially with a switch in the asymmetry between the Hsp90 monomers, on the way to a more compact ADP state. Release of the nucleotide returns Hsp90 back to the equilibrium of open states. Inhibitors, like geldanamycin, 17AAG, radicicol, etc break the ATPase cycle of Hsp90, stalling it in the more compact state, yet different from the ATP bound state.
