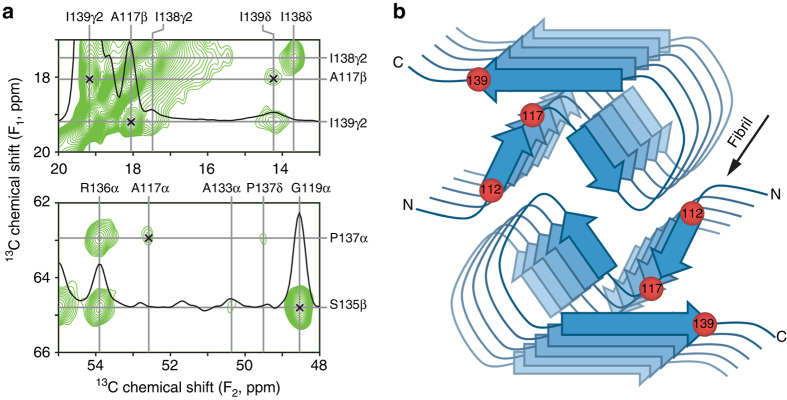
Fig. 2.
Key interresidue contacts and schematic model of the human PrP23-144 amyloid β-core. a Small regions of a 900 MHz two-dimensional 13C–13C DARR solid-state NMR spectrum recorded with a mixing time of 500 ms for amyloid fibrils generated from huPrP23-144 expressed with 3-13C-pyruvate as the carbon source. The spectral regions contain the key restraints on the [hu] amyloid core structure in the form of unambiguous long-range correlations (indicated by x-marks) between the following 13C atoms: A117Cβ-I139Cγ2, A117Cβ-I139Cδ, A117Cα-P137Cα, and G119Cα-S135Cβ. b Schematic model for the [hu] amyloid core based on the combination of solid-state NMR and tilted-beam transmission electron microscopy data (see text for details). In this model [hu] amyloid fibrils consist of two protofilaments in a C2-symmetric arrangement with β-sheet regions running parallel to the long fibril axis. The approximate locations of amino acid residues 112, 117, and 139, that have major impact on the structure adopted by PrP23-144 amyloid as discussed in the text, are indicated by red spheres