Fig. 3.
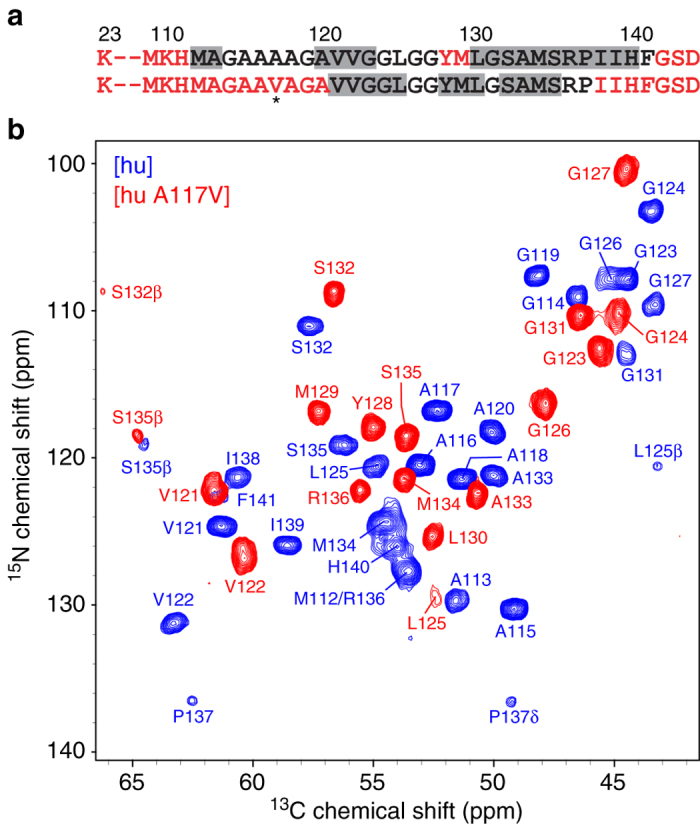
Two-dimensional 15N–13Cα solid-state NMR spectrum of amyloid fibrils formed by the A117V mutant of human PrP23-144. a Amino acid sequences of huPrP23-144 and huPrP23-144 A117V, with the mutation site indicated by an asterisk. Immobile residues located within the amyloid core and conformationally flexible residues are shown in black and red fonts, respectively. Residues with the highest β-strand propensity based on TALOS-N41 analysis are indicated by gray rectangles (c.f., Fig. 1 and Supplementary Fig. 5). b Assigned two-dimensional 15N–13Cα fingerprint solid-state NMR spectrum of [hu A117V] amyloid fibrils (red contours), overlaid with the corresponding spectrum of [hu] amyloid (blue contours)
