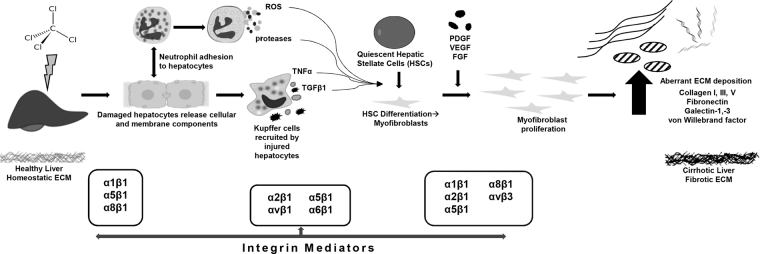
Figure 2.
Schematic of aberrant ECM accumulation following CCl4 injury. Key extracellular matrix proteins (ECMPs) and cognate integrin receptors in CCl4 exposure mouse model of fibrosis. The phenomena include quiescent hepatic stellate cell (HSC) activation and their subsequent differentiation into myofibroblasts after which growth factor-induced proliferation leads to the aberrant ECM deposition that characterizes cirrhotic liver fibrosis. The chronic inflammatory response involves impaired matrix degradation which further contributes to dyshomeostasis of ECM proteins, and therefore tissue structure and errant signal transduction. Following exposure to CCl4, damaged hepatocytes release cellular and membrane components8, leading to recruitment of neutrophils and Kupffer cells. Profibrogenic and proinflammatory cytokines, reactive oxygen species (ROS), and proteases are released from resident immune cells, leading to stimulation and activation of quiescent HSCs, inducing their differentiation to myofibroblasts. Proliferation of activated myofibroblasts in response to fibrogenic factors results in excessive ECM deposition, leading to fibrotic scarring and end-stage liver disease. Integrin mediators known to be active in fibrotic pathology include β1, α1, α5, and α6 on hepatocytes, which correlate clinically with stage of fibrosis8. αvβ3 integrin signaling from HSCs/myofibroblasts is involved with regulating ECM-fibrolytic matrix metalloproteinases. De novo α8β1 expression in activated HSCs occurs in response to CCl4 injury; likewise, α1, α2, and α5 on HSCs is indicative of activation, enhancing attachment to basement membrane proteins8. Feed forward mechanism results from the fibrillar ECM itself enhancing HSC activation, implicating integrins α1β1, α2β1, and αVβ1 33.