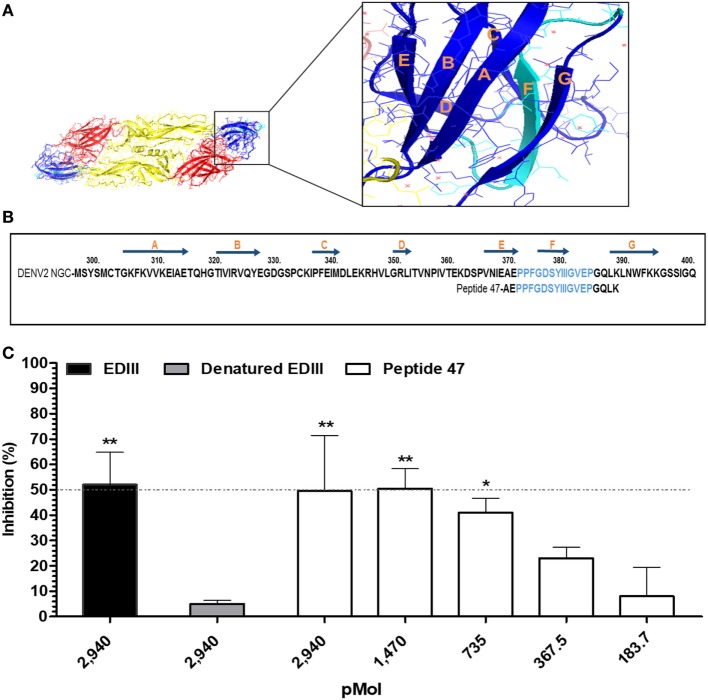
Figure 4.
Identification of a linear envelope glycoprotein domain III (EDIII)-derived epitope involved in binding of DENV2 to host cells. (A) 3D representation of DENV E protein dimer. Domains I (yellow), II (red), and III (blue) are highlighted. The insert displays EDIII structural features. The amino acid sequence marked in light blue encompasses the epitope recognized by antibodies raised in mice immunized with labile toxins (LT)-adjuvanted vaccines. (B) Amino acid sequence of DENV2 EDIII (GenBank accession number: AF038403.1). The positions of the EDIII β strands are indicated above the sequence by dark blue arrows, while the sequences of the synthetic peptide used in the competition assays are shown below. The epitope predicted by the immunosignature is marked in light blue. (C) Inhibition of DENV2 infection. Different molar concentrations of synthetic peptide 47 were mixed with DENV2 New Guinea C and subsequently added to cells. Purified EDIII and heat-denatured EDIII were used as positive and negative controls in the inhibition of virus infectivity assay, as indicated in the figure. Cells stained with primary mAb 4G2 and subsequently with Alexa 488-conjugated anti-mouse IgG were measured by cytometry, and the number of DENV-positive infected Vero cells was determined for each group. Values express the reduction in the percentage of the number of virus-infected cells compared to the control group denatured EDIII- (untreated DENV2). Dashed line indicates 50% inhibition of virus infection. Bars represent the mean values and SEM from three independent experiments. ***p < 0.001, **p < 0.001, and *p < 0.05 represent significant differences with regard to denatured EDIII-treated and untreated DENV2 (two-way ANOVA with Bonferroni post hoc test).