Abstract
This study sought to investigate the anti-inflammatory effect of Polyene Phosphatidylcholine (PPC), a clinical drug that is used to treat hepatopathy, on lipopolysaccharide (LPS)-stimulated macrophages and on bovine collagen II-induced arthritis (CIA) rats. In stimulated primary and Raw264.7 macrophages by LPS, PPC significantly down-regulated the relative expression of mRNA such as IL-6, TNF-α, TLR-2, TLR-4, MyD88, and NF-κB while up-regulated IL-10 and TGF-β expression. Moreover, the concentration of IL-6, TNF-α, IL-10, and TGF-β in the cultured supernatants showed the similar tendency with their mRNA alterations. In addition, PPC could significantly inhibit the LPS-induced expression of MyD88 and NF-κB p65 in both mRNA and protein levels. These results suggest that PPC could down-regulate the LPS-stimulated inflammation in macrophages through TLR-2/TLR-4/MyD88/NF-κB pathway in vitro. Furthermore, to explore its effects in vivo, PPC was administrated to CIA rats. In comparison to CIA group, PPC-treated rats showed decreased arthritis score and osteopenia. Besides, PPC exhibited its ability to alleviate the degree of synovial hyperplasia, inflammatory cell infiltration, and destruction of cartilage and bone, thus remarkably improving the condition of CIA rats. In short, this study demonstrated that PPC had the potential to be an anti-inflammatory drug to treat inflammatory disorders such as rheumatoid arthritis.
Keywords: Polyene Phosphatidylcholine, collagen-induced arthritis, macrophages, toll-like receptor
Introduction
Rheumatoid arthritis (RA) is one of the most prevalent autoimmune inflammatory diseases worldwide and is characterized by the infiltration of inflammatory cells and proliferation of fibroblasts in the synovial joints, leading to chronic inflammation and progressive destruction of bone and cartilage [1]. Its pathogenesis is a very complex process involving genetic factors, environmental factors and systemic immune responses [2-4], among which chronic synovial inflammation has been shown to play an essential role in sustainability of the disease [5]. Therefore, down-regulation of synovial inflammation is believed to be an effective way to treat RA.
Synovial tissues contain many types of immunocytes, including synovial fibroblasts (SF), macrophages, dendritic cells, T cells and B cells [6]. The immunocytes augment abundantly during active disease, and their activation has been shown to be the key events that trigger and maintain the inflammatory response [7,8]. Toll-like receptors (TLRs), as one of the conserved pathogen-associated molecular patterns (PAMPs), play an essential role in this process, revealed by studies in both human and mouse models of RA [9,10]. Previous studies believed that TLRs are driven by inflammation in response to TLR ligands of microbial origin. However, more recently endogenous TLR ligands have been found in the joints or sera of RA patients and their levels have been correlated with disease activity scores [11,12]. TLRs therefore have been recognized as key contributors to the pathogenesis of the disease. Hence, there is an increasing interest in targeting TLRs to treat the disease [13]. For example, Abdollahhi-Roodsaz and colleagues had showed that the TLR-4 antagonist had an impressive therapeutic effect in collagen-induced arthritis (CIA) mice [14].
Polyene Phosphatidylcholine (PPC) is extracted from soy and rich in polyunsaturated fatty acid, such as linoleic acid, linolenic acid, and oleic acid. It has been widely used to treat various types of hepatopathy clinically [15], including alcohol-induced hepatic fibrosis [16], hepatocyte steatosis [17], and nonalcoholic steatohepatitis (NASH) [18]. Phosphatidylcholine (PC) is the main ingredient of PPC, and is an important component of cytomembrane and organelle membrane. In the past decades, PC had been utilised for protecting health, with its function of nourishing the brain, beautifying the features, reducing weight, and scavenging blood vessels, even viewed as the third nutriment behind protein and vitamine. Interestingly, in recent years, it was reported that several conjugates containing PC could improve the condition of RA [19] and inflammatory bowel disease (IBD) [20], which suggest that PC could act as an anti-inflammatory drug. Thus, we set out to determine the therapeutic effects of PPC on RA. PPC may be easier to practice in clinic, because it has been extensively applied for auxiliary hepatinica treatment for a long time and thus owns a reliable assurance for patients. Nevertheless, it remains obscure whether PPC can treat RA and the possible underlying molecular mechanisms are also unclear.
The present work was to investigate whether PPC could inhibit the inflammatory response in LPS-stimulated macrophages and in CIA rats via TLRs/MyD88/NF-kB pathway. We hope, these results can provide a theoretical basis for the application of PPC in autoimmune and inflammatory diseases including RA.
Materials and methods
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM, GIBCO, USA), fetal bovine serum (FBS, GIBCO, USA), streptomycin and penicillin (GIBCO, USA), TRIzol (Life Technologies, USA), CCK-8 (Dojindo, Shanghai, China), Annexin V-PE Apoptosis Detection Kit (eBioscience, USA) and mouse ELISA kits (eBioscience, USA), Freund’s complete adjuvant (Sigma, USA), LPS (Sigma, USA), PrimeScript™ RT Master Mix (Takara, Japan), SYBR® Premix Ex Taq™ (Takara, Japan), Monoclonal antibodies (MAb) against NF-κB p65 and MyD88 (Abcam, USA), β-actin (Abcam, USA), secondary horseradish peroxidase (HRP)-conjugated antibody (Abcam, USA), Bovine collagen type II (Chondrex, USA), Polyene Phosphatidylcholine (Sanofi Aventis, Spain).
Cell line culture
Mouse macrophage cell line Raw264.7 was obtained from our laboratory and were cultured in DMEM supplemented with 10% FBS, 100 μg/mL streptomycin, 100 U/mL penicillin. Mouse primary peritoneal macrophages were prepared from female C57B/6 mice (6-8 weeks of age) as described [21]. After 2 h, non-adherent cells were removed and the adherent cells were used as peritoneal macrophages. Cell cultures were maintained at 37°C in a humidified atmosphere (5% CO2).
Detection of cell apoptosis by flow cytometry assay
Raw264.7 cells were treated with indicated concentrations of PPC for 24 h with or without LPS (100 ng/ml), and then harvested and labeled with PE-Annexin V and 7-Amimo-Actinomycin (7-AAD) (Lianke company, China) following the manufacturer’s instructions. Stained cells were determined by FACS Canto II flow cytometer (BD, Biosciences, USA) and data were analyzed using FlowJo software.
Cell proliferation assay
A cell counting kit (CCK-8) was used to evaluate cell proliferation according to the manufacturer’s instructions. Raw264.7 cells were seeded in 96-well plates at a density of 2×103/well in 100 μl volume. PPC was added in different concentrations (0, 1, 2, 5 and 10 μM). At the point of 20, 44 and 68 h, CCK reagent was added into the medium (10 μl/well). The optical density of each well was determined at 450 nm after 4 h of incubation using a Synergy 2 Microplate Reader (Bio-Tek, USA).
Enzyme-linked immunosorbent assays (ELISA)
The levels of IL-6, TNF-α, TGF-β and IL-10 in cultured cell supernatants were determined using ELISA kits (eBioscience, USA) according to manufacturer instructions.
Quantitative real-time RT-PCR
RNA was extracted from Raw264.7 cells and peritoneal macrophages using TRIzol reagent, and cDNA was synthesized from the RNA using PrimeScript™ RT Master Mix. Following reverse transcription, cDNA was amplified using SYBR® Premix Ex Taq™ with gene-specific primers. Quantitative PCR analyses were performed in a LightCycler® 480 II detection system (Roche Applied Science, Penzberg, Germany) under the following thermal cycler conditions: one cycle of 5 min denaturation at 95°C, and then 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C for 45 cycles using the primers listed in Table 1. All experiments were performed in triplicate and the Ct values were normalized to endogenous reference (GAPDH). The relative expression of detection indexes of this manuscript was indicated by comparative cycling threshold (Ct) normalized by GAPDH with the 2-ΔΔCt method.
Table 1.
Primer sequences used for quantitative real-time PCR analysis
| Gene | Primer sequences |
|---|---|
| TNF-α | F-CATCTTCTCAAAATTCGAGTGACAA |
| R-TGGGAGTAGACAAGGTACAACCC | |
| IL-6 | F-CCACGGCCTTCCCTAC |
| R-AAGTGCATCATCGTTGT | |
| TGF-β | F-CTGGATACCAACTACTGCTTCAG |
| R-TTGGTTGTAGAGGGCAAGGACCT | |
| IL-10 | F-GCTCCAGAGCTGCGGACT |
| R-TGTTGTCCAGCTGGTCCTTT | |
| TLR-2 | F-TGTCTCCACAAGCGGGACTT |
| R-TTCGATGGAATCGATGATGTTG | |
| TLR-4 | F-TGACAGGAAACCCTATCCAGAGTT |
| R-TCTCCACAGCCACCAGATTCT | |
| MyD88 | F-AAGAAAGTGAGTCTCCCCTC |
| R-TCCCATGAAACCTCTAACAC | |
| NF-κB | F-AGCACAGATACCACCAAGAC |
| R-TCAGCCTCATAGTAGCCATC | |
| GAPDH | F-CAACTTTGGCATTGTGGAAGG |
| R-ACACATTGGGGGTAGGAACAC |
Western blotting analysis
Total protein was extracted from Raw264.7 cells and analyzed with bicinchoninic acid protein concentration assay kit (Beyotime Biotech, Beijing, China). Sample protein was separated by electrophoresis in 10% SDS-PAGE with a Bio-Rad electrophoresis system (Hercules, CA, USA). The primary antibodies (rabbit NF-κB p-65, MyD88 antibody, Abcam, UK, 1:1000 dilutions) were incubated at 4°C 24 h. The secondary anti-bodies (anti-rabbit IgG, 1:2000 dilutions) were incubated for 2 h at room temperature. The membrane containing antibody-protein complexes were visualized with an enhanced chemiluminescence detection system on radiograph film (Bio-rad, Hercules, CA, USA). The bands were scanned and analyzed by the software Quantity ONE (Bio-rad, Hercules, CA, USA). The expression of protein in each sample was normalized by β-actin.
Animals
Female SD rats (125-150 g, 6-8 weeks of age) were purchased from the Animal Center of Xuzhou Medical University (Xuzhou, Jiangsu). All rats were housed in an air-conditioned room at 24°C with a 12 h dark/light cycle and permitted free access to standard laboratory food and water.
CIA model establishment and groups
SD rats were randomly divided into 3 groups with 10 rats in each group: CIA group; PPC treatment group (100 µg/rat) and control group without being modeled. 2.5 ml Bovine collagen type II (5 mg) was dissolved in 2.5 ml Freund’s complete adjuvant, and finally made into an emulsifiable liquid of 1 mg/ml. For arthritis induction, rats were anaesthetized and subcutaneously injected with 100 μl of bovine collagen type II (CII) on day 1 and day 10. Controls received equal volume of Freund’s complete adjuvant in the same time. PPC, at a dose of 100 µg/rat, was administered to CIA rats by tail vein injection on days -1 (the day before the first immunization) and 9.
Arthritis index
The evaluation of arthritis index (AI) mainly involved the hind ankle joints. Four points were selected for each of the hind legs (0 point, no inflammation and joint swelling; 1 point, red spots or mild swelling; 2 points, moderate joint swelling; 3 points, severe joint swelling; 4 points, joint rigidity, deformity or severe dysfunction), and total 8 points for each animal. The degree of joint welling was measured on the basis of the diameter of the right ankle with a vernier caliper.
Pathologic evaluation
All animals were sacrificed 42 d after primary immunization. Bilateral hind ankles were fixed with 10% neutral formalin for 48 h, and decalcified with 10% EDTA for 3 weeks. After that, ankles were incised longitudinally, embedded in paraffin. HE staining was used to determine synovial hyperplasia and inflammatory infiltration in the knee joints, and Safranin O-fast green staining was used to evaluate the cartilage degradation while commercial tartrate-resistant acid phosphatase (TRAP) kit was to observe the osteoclastogenesis.
Statistical analysis
All results were presented as mean ± SD. All statistical analysis was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla, CA, USA). Assessments of significant differences comparing multiple groups were performed by one-way or two-way ANOVA. A P-value <0.05 implied significance.
Results
PPC does not induce apoptosis and inhibit growth of Raw264.7 cells
We first tested the apoptotic sensitivity of PPC to Raw264.7 cells in order to determine its cytotoxicity. Figure 1A showed that none of the examined concentrations of PPC (up to 10 μM) induced any apoptosis in LPS-activated Raw264.7 cells at 24 h compared with LPS group (P<0.05). Furthermore, we evaluated whether PPC played an inhibitory role in the growth of Raw264.7 cells using CCK8 assay. The absorbance values showed that PPC did not reduce the cell viability of Raw264.7 cells within 3 days (Figure 1B, P>0.05). These results suggest that PPC has no cytotoxic effects on Raw264.7 cells.
Figure 1.
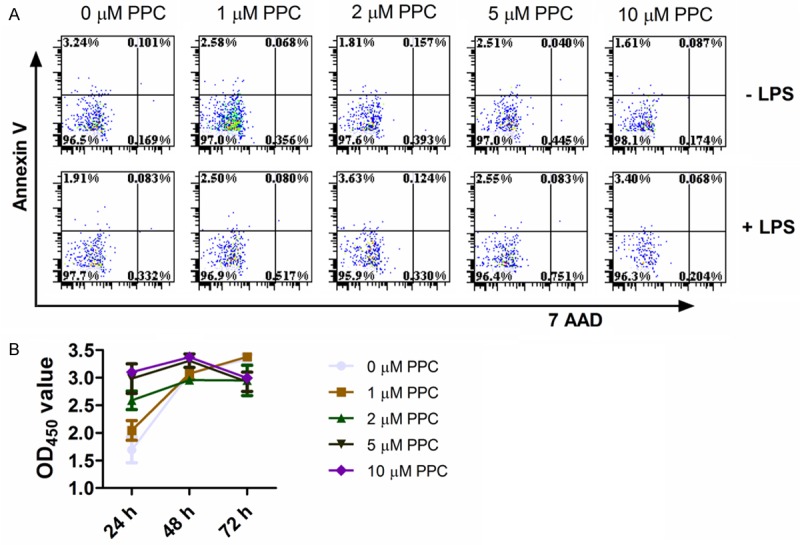
The effect of PPC on the apoptosis and proliferation of Raw264.7 cells. A: The Raw264.7 cells were exposed to mentioned concentrations of PPC in the absence or presence of LPS (100 ng/ml) for 24 h, and then the apoptosis profiles were determined by FACS. B: The Raw264.7 cells were exposed to mentioned concentration of PPC for 24, 48, 72 h, and then their proliferation were monitored by CCK-8. Data are shown as mean ± SD of three independent experiments. Comparisons among multiple groups were done using two-way ANOVA. *, P<0.05, **, P<0.01.
PPC inhibits the release of pro-inflammatory cytokines induced by LPS in macrophages
Macrophages are well known to play a crucial role in the regulation of inflammatory response. Therefore, this study used Raw264.7 cells and primary macrophages as a model to investigate the anti-inflammatory function of PPC. The effects of PPC on the expression of cytokines, no matter at the level of mRNA or protein, both in LPS-stimulated Raw264.7 cells and primary macrophages were evaluated. As shown in Figure 2A, the mRNA expression of TNF-α and IL-6 in Raw264.7 cells were significantly decreased in LPS plus PPC group compared with LPS stimulated group (P<0.05). These results show that PPC could potently inhibit LPS-induced production of pro-inflammatory cytokines IL-6 and TNF-α. With regards to anti-inflammatory factors, however, the mRNA expression of TGF-β and IL-10 were significantly increased (P<0.05). Moreover, the expression of these cytokines at protein level shared the similar tendency with their mRNA alternations (Figure 2C). In addition, PPC also inhibited IL-6 and TNF-α production while concurrently promoting IL-10 production in primary macrophages (Figure 2B). Overall, PPC can down-regulate the inflammatory response to LPS.
Figure 2.
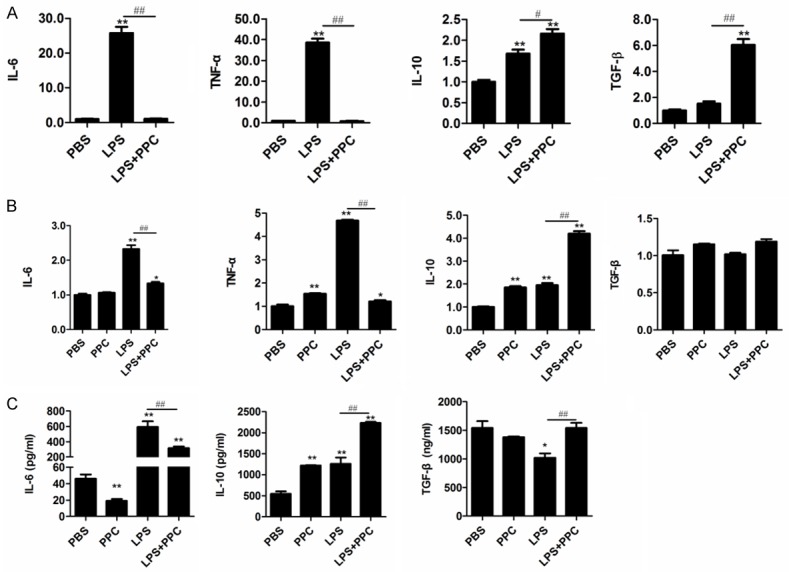
The effect of PPC on LPS-triggered cytokine production in Raw264.7 cells and mouse primary macrophages. Cells were stimulated by PPC (5 µM) with or without LPS (100 ng/ml) for 24 h. The mRNA expression of IL-6, TNF-α, IL-10, and TGF-β in Raw264.7 cells (A) and mouse primary macrophages (B) were examined by Real time RT-PCR. The concentrations of IL-6, TGF-β, and IL-10 in the supernatants were measured by ELISA (C). Data are shown as mean ± SD of three independent experiments. Comparisons among multiple groups were done using one way ANOVA. PPC, LPS, LPS+PPC vs PBS, *, P<0.05, **, P<0.01; LPS vs LPS+PPC, #, P<0.05, ##, P<0.01.
PPC inhibits the activation of TLR-2/TLR-4/MyD88/NF-κB pathway stimulated by LPS in the macrophages
TLR-4/TLR-2 ligation leads to recruitment of its downstream signaling pathways including the adapter protein MyD88 and transcription factor NF-κB, which ultimately result in the production of pro-inflammatory cytokines. Hence, this study examined the expression of TLRs/MyD88/NF-κB both at mRNA and protein levels. As shown in Figure 3A, the mRNA expression of TLR-2, TLR-4, MyD88 and NF-κB in LPS stimulated Raw264.7 cells were significantly reduced by PPC (P<0.05). Moreover, a similar regulatory role of PPC on these genes in mouse primary macrophages was also observed (Figure 3B, P<0.05). In addition, western blot analysis (Figure 3C) also showed that PPC could reduce LPS-induced activation of MyD88 and NF-κB p65. Therefore, these results suggest that PPC can decrease LPS-induced activation of MyD88 and NF-κB signaling pathway triggered by TLR-2/TLR-4 in macrophages.
Figure 3.
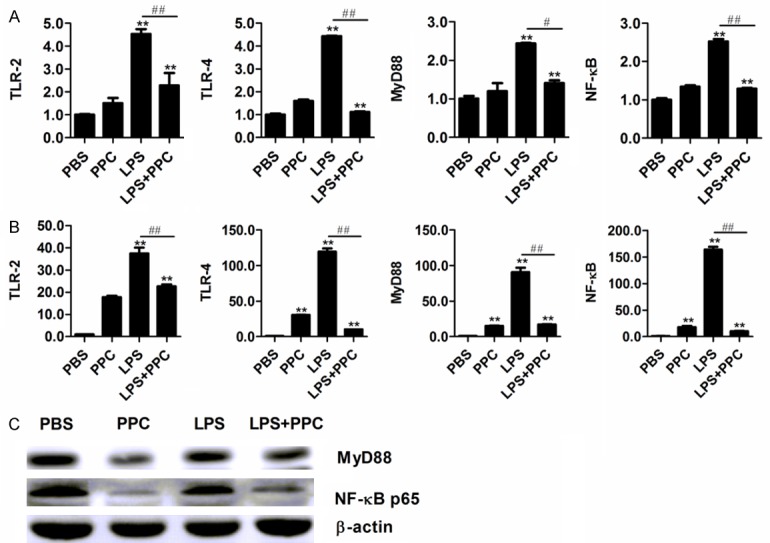
The inhibitory role of PPC on the activation of TLRs/MyD88/NF-κB pathway induced by LPS in Raw264.7 cells and mouse primary macrophages. The cells were stimulated with LPS (100 ng/ml) with or without PPC (5 μM) for 24 h. The mRNA expression of TLR-2, TLR-4, MyD88, and NF-κB were detected by Real time RT-PCR in Raw264.7 cells (A) and in mouse primary macrophages (B). The protein levels of MyD88, NF-κB p65, and β-actin in Raw264.7 cells were determined by Western blot (C). Data are shown as mean ± SD of three independent experiments. Comparisons among multiple groups were done using one way ANOVA. PPC, LPS, LPS+PPC vs PBS, *, P<0.05, **, P<0.01; LPS vs LPS+PPC, #, P<0.05, ##, P<0.01.
PPC effectively ameliorates the condition of rat CIA
Significant swellings of CIA groups had been mimicked in the right and left knee joints (Figure 4A). Both arthritis score and the thickness of paws and ankles in PPC treatment group were significantly lower compared with those of CIA group (Figure 4A, P<0.05). The erythema and swelling in the toes and ankles of rats were significantly lessened (Figure 4B) after PPC treatment. Moreover, the loss of sclerotin, destruction of structural integrity and narrowing of the joint space were also improved after PPC treatment (data not shown).
Figure 4.
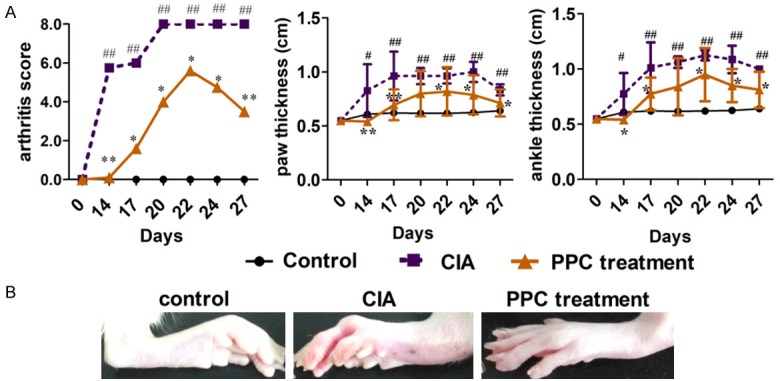
The theraputic effect of PPC on rat CIA. Bovine CII was subcutaneously injected to induce the arthritis model on day 1 and day 10. Controls received only Freund’s complete adjuvant in the same time. 100 µg PPC was intravenously administered to each CIA rat on day 1 and day 9. The arthritis scores, the thicknesses of rat paw and ankle thickness (A) were evaluated from day 0 to day 27 (n=10 for each group). An example of improved condition of CIA rat joints was presented in (B). Comparisons among multiple groups were done using two way ANOVA. Control group vs CIA group, *, P<0.05, **, P<0.01. PPC treatment group vs CIA group, #, P<0.05, ##, P<0.01.
HE staining showed that articular surface of rats in the control group were smooth, and there weren’t any infiltration inflammatory cells in the articular cavity (Figure 5A). However, the abnormal hyperplasia was identified, accompanying with a large number of inflammatory cells infiltration, in the synovial tissues of rats from the CIA group (Figure 5B), while both of which were attenuated when injecting PPC (Figure 5C). Safranin O-fast green is able to put color on the articular cartilage. Compared with the control group (Figure 5D), the articular cartilage of CIA rat became less and thin, and the articular surface showed wide destruction and was asperous (Figure 5E). However, this situation was significantly ameliorated by PPC treatment (Figure 5F). TRAP staining showed that multiple TRAP-positive cells were accumulated along the impaired articular surface in CIA rats (Figure 5H), and their numbers were remarkably reduced via administrating PPC (Figure 5I), which indicated that PPC inhibited the differentiation of osteoclasts. Overall, these results show the effective protection of PPC on the joint destruction, improving the condition of CIA rats.
Figure 5.
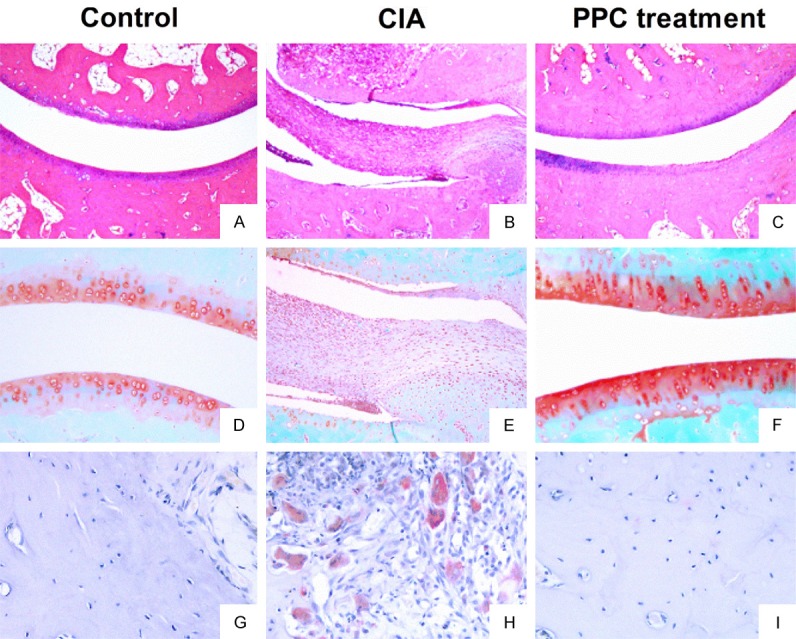
The pathological manifestations of CIA rats after PPC treatment. All animals were sacrificed at day 42 after primary immunization. Ankle joints were harvested for evaluating the pathological changes. A-C: Showed the representative results of HE staining. A: The articular surface was smooth without any infiltration of inflammatory cells in the articular cavity of control group. B: The infiltration of inflammatory cells and the unlimited proliferation of SF cells in the articular cavity of CIA rats. C: The articular surface was smooth without invading SF cells after PPC treatment. D-F: Showed the results of Safranin O-fast green staining. D: The bone structure and cartilage of control group were intact in articular cavity. E: The articular cartilage encountered serious destruction in CIA rats. F: The condition of destruction was ameliorated by PPC treatment. G-I: Showed the results of TRAP staining. G: Only a few osteoclasts were found in control joints. H: The numbers of osteoclasts were significantly increased in the joints of CIA rats. I: The numbers of osteoclasts were obviously decreased after PPC treatment.
Discussion
The immunocytes infiltrating in the RA joint produce a large number of cytokines, such as TNF, IL-1 and IL-6, thus creating the inflammatory environment [22,23]. Biological therapies such as the utilization of TNF antibody and IL-6 receptor antibody to treat RA are being recommended in recent years [24,25]. Although there are improvements in many aspects by the use of these methods, some shortcomings indeed exist. For instance, many patients, rather than achieving an expected curative effect adequately after anti-TNF treatment, may only become unresponsive to this treatment over time. Moreover, the fancy price of biological therapies remains a factor that severely restricts their use. Therefore, current studies should still insist on developing novel therapeutic approaches to RA treatment. This study mainly evaluated the anti-inflammatory effects of PPC on LPS-stimulated macrophages and on CIA rats to explore its potential to be a drug candidate against RA.
PPC did not obviously affect the growth and apoptosis of Raw264.7 cells, suggesting a weak cytotoxicity to normal cells. Actually, the safety is obvious, because the drug has been widely applied to treat liver diseases for many years. Also, PC is the fundamental substance of cell and is often recommended as health remedy. Thus, PPC was proposed to have considerable exploration potential to be an ideal anti-inflammatory drug.
PPC could inhibit the release of pro-inflammatory cytokines (TNF-a, IL-6) stimulated by LPS and boost the production of anti-inflammatory cytokines (IL-10, TGF-β). Previous studies had illustrated that joint damage of RA patients was mainly caused by the infiltration of mononuclear cell, proliferation of synovial fibroblasts, and the accumulation of matrix metalloproteinases and proinflammatory cytokines [26,27]. On one hand, TNF-α and IL-6 could promote progressive destruction of cartilage and bone, regulate synovial hyperplasia, and mediate bone resorption via enhancing the synthesis of rheumatoid factor [28-30]. On the other, the anti-inflammatory cytokines IL-10 and TGF-β limit the inflammatory response by inhibiting pro-inflammatory cytokines, suppressing the differentiation of Th1 cells and inducing regulatory T cells, however, the levels of these anti-inflammatory cytokines were declined in the active stage of RA [31-34]. Therefore, it is important to control the disease progression by regulating the production of the cytokines. In brief, the present study showed the regulatory function of PPC on Raw264.7 cells and primary macrophages, which seemed to be one reason for ameliorating the severity of CIA rats.
Moreover, PPC was also found to inhibit LPS-induced activation of TLR-2 and TLR-4/MyD88/NF-κB pathway which participate in the whole process of RA. Macrophages from patients in the active stage of RA could express higher levels of TLR-2 and secrete more inflammatory cytokines than those from inactive state [35,36], which result in exacerbation of arthritis. Similarly, TLR-4 could accelerate joint inflammation and bone erosion [37]. Notably, increasing studies demonstrated that many endogenous TLR ligandsarose at different stages of RA [38], which revealed the key contributions of TLRs to the inflammation [39]. In fact, targeting TLR-2 and TLR-4 have showed an attractive therapeutic effect on CIA mouse models and RA patients [40]. A previous study had reported that PC exclusively interacted with TLR-2 and TLR-4 [41]. Our study found that PPC effectively downregulated the expression of TLR-2 and TLR-4 in LPS stimulated macrophages.
Another pro-inflammation mechanism involved in RA is the activation of MyD88 and NF-κB, which strongly increases the expression of pro-inflammatory cytokines and chemokines [42]. MyD88, the central adaptor molecule interacting with all TLRs except TLR3, is involved in the spontaneous production of cytokines in RA [43]. Moreover, factor(s) released from synovial membrane cultured from RA tissues could stimulate macrophages in a MyD88-dependent way [44]. This study found that PPC significantly inhibited the expression of MyD88. Furthermore, PPC was also demonstrated to reduce the expression of NF-κB, which was also observed in the liver tissue of NASH rat [17]. Consequently, the inhibitory effect of PPC on the LPS-induced inflammatory cytokines might pass through down-regulating the TLR2/TLR4/MyD88/NF-κB pathway.
RA patients present some features including persistent infiltration of leukocytes, synovial membrane hyperplasia and destruction of bone and cartilage [45,46], which are all important factors of RA pathological and clinical manifestations. Aggressive infiltration of leukocytes in rheumatoid synovial tissue produces large amounts of cytokines and chemokines, and even causes synoviocyte hyperplasia, invades cartilage, and finally lead to the destruction of bone and cartilage. Synoviocyte hyperplasia results in pannus formation and damage of the joints [47] while osteoclast activation mediates abundant bone resorption and destroys bone and cartilage, ultimately resulting in malformation and even dysfunction of joints [46]. Pathological observation of our study revealed that PPC could potently alleviate the condition of CIA rats by decreasing inflammatory cell infiltrate, ameliorating synovial hyperplasia and progressive destruction of articular cartilage.
In summary, this study showed that PPC, the classic hepatinica in clinic, inhibited LPS-induced inflammation in macrophages via down-regulating TLR-2/TLR-4/MyD88/NF-κB pathway in vitro, and thus improving the condition of CIA rats. These results indicate that PPC could act as an anti-inflammatory drug to treat inflammatory diseases, including RA.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81501762), the Starting Foundation for Talents of Xuzhou Medical College (No. D2015004), the Natural Science Foundation of the Jiangsu Higher Education Institutions (No. 15KJB310025), the Jiangsu Planned Projects for Postdoctoral Research Funds (No. 1501061A), the China Postdoctoral Science Foundation funded project (No. 2015M581864), Jiangsu Provincial Natural Science Foundation of China (No. BK20151168), Xuzhou Technology Bureau Foundation (KC14SH074), the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 2014 To RXT), and the Jiangsu Qing Lan Project. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
Authors’ contribution
Conceived and designed the experiments: WP, KYZ, RXT. Performed the experiments: WP, WTH, HWX, XYL, XML, FFS, HL. Analyzed the data: WP, WTH, XYL, FFS. Contributed reagents/materials/analysis tools: SPQ, RXT. Wrote the manuscript: WP, WTH.
References
- 1.Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 2.McGarry T, Biniecka M, Gao W, Cluxton D, Canavan M, Wade S, Wade S, Gallagher L, Orr C, Veale DJ, Fearon U. Resolution of TLR2-induced inflammation through manipulation of metabolic pathways in rheumatoid arthritis. Sci Rep. 2017;7:43165. doi: 10.1038/srep43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizuno M, Noto D, Kaga N, Chiba A, Miyake S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One. 2017;12:e0173032. doi: 10.1371/journal.pone.0173032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mclnnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 5.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki Y, Mimura T. The mechanisms underlying chronic inflammation in rheumatoid arthritis from the perspective of the epigenetic landscape. J Immunol Res. 2016;2016:6290682. doi: 10.1155/2016/6290682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill LA. How Toll-like receptors signal: what we know and what we don’t know. Curr Opin Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Goh FG, Midwood KS. Intrinsic danger: activation of Toll-like receptors in rheumatoid arthritis. Rheumatology (Oxford) 2012;51:7–23. doi: 10.1093/rheumatology/ker257. [DOI] [PubMed] [Google Scholar]
- 10.McCormack WJ, Parker AE, O’Neill LA. Toll-like receptors and NOD-like receptors in rheumatic diseases. Arthritis Res Ther. 2009;11:243. doi: 10.1186/ar2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein RS, Bruchfeld A, Yang L, Qureshi AR, Gallowitsch-Puerta M, Patel NB, Huston BJ, Chavan S, Rosas-Ballina M, Gregersen PK, Czura CJ, Sloan RP, Sama AE, Tracey KJ. Cholinergic anti-inflammatory pathway activity and high mobility group box-1 (HMGB1) serum levels in patients with rheumatoid arthritis. Mol Med. 2007;13:210–215. doi: 10.2119/2006-00108.Goldstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM 2nd, Tak PP, Haines GK 3rd, Nicchitta CV, Pope RM. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182:4965–4973. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joosten LA, Abdollahi-Roodsaz S, Dinarello CA, O’Neill L, Netea MG. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol. 2016;12:344–357. doi: 10.1038/nrrheum.2016.61. [DOI] [PubMed] [Google Scholar]
- 14.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, van der Meer JW, Netea MG, van den Berg WB. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56:2957–2967. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Zhang XJ, Lan Y, Xu L, Zhang XZ, Wang HH. Treatment of non-alcoholic fatty liver disease by qianggan capsule. Chin J Integr Med. 2010;16:23–27. doi: 10.1007/s11655-010-0023-1. [DOI] [PubMed] [Google Scholar]
- 16.Lieber CS, Robins SJ, Li J, DeCarli LM, Mak KM, Fasulo JM, Leo MA. Phosphatidylcholine protects against fibrosis and cirrhosis in the baboon. Gastroenterology. 1994;106:152–159. doi: 10.1016/s0016-5085(94)95023-7. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Chen DF. Effect of polyene phosphatidyl choline on hepatocyte steatosis via PPARα/CPT-1A pathway. Zhonghua Gan Zang Bing Za Zhi. 2016;24:291–296. doi: 10.3760/cma.j.issn.1007-3418.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Cao M, Li X, Zhang B, Han S, Yang Y, Zhou B, Zhang Y. The effect of polyene phosphatidyl choline intervention on nonalcoholic steatohepatitis and related mechanism. Am J Transl Res. 2016;8:2325–2330. [PMC free article] [PubMed] [Google Scholar]
- 19.Bashi T, Shovman O, Fridkin M, Volkov A, Barshack I, Blank M, Shoenfeld Y. Novel therapeutic compound tuftsin-phosphorylcholine attenuates collagen-induced arthritis. Clin Exp Immunol. 2016;184:19–28. doi: 10.1111/cei.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Ami Shor D, Bashi T, Lachnish J, Fridkin M, Bizzaro G, Barshak I, Blank M, Shoenfeld Y. Phosphorylcholine-tuftsin compound prevents development of dextransulfate-sodium-salt induced murine colitis: implications for the treatment of human inflammatory bowel disease. J Autoimmun. 2015;56:111–117. doi: 10.1016/j.jaut.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14:Unit 14.1. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol. 2017;13:217–233. doi: 10.1038/nrrheum.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Maas A, Kievit W, van den Bemt BJ, van den Hoogen FH, van Riel PL, den Broeder AA. Down-titration and discontinuation of infliximab in rheumatoid arthritis patients with stable low disease activity and stable treatment: an observational cohort study. Ann Rheum Dis. 2012;71:1849–1854. doi: 10.1136/annrheumdis-2011-200945. [DOI] [PubMed] [Google Scholar]
- 25.Emery P, Keystone E, Tony HP, Cantagrel A, van Vollenhoven R, Sanchez A, Alecock E, Lee J, Kremer J. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biological: results from a 24-week multicentre randomized placebo-controlled trial. Ann Rheum Dis. 2008;67:1516–1523. doi: 10.1136/ard.2008.092932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi F, Zhou D, Ji Z, Xu Z, Yang H. Anti-arthritic activity of luteolin in Freund’s complete adjuvant-induced arthritis in rats by suppressing P2X4 pathway. Chem Biol Interact. 2015;226:82–87. doi: 10.1016/j.cbi.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Szekanecz Z, Koch AE. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:5–13. doi: 10.1038/nrrheum.2015.157. [DOI] [PubMed] [Google Scholar]
- 28.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozgen M, Koca SS, Karatas A, Dagli AF, Erman F, Gundogdu B, Sahin K, Isik A. Lapatinib ameliorates experimental arthritis in rats. Inflammation. 2015;38:252–259. doi: 10.1007/s10753-014-0028-6. [DOI] [PubMed] [Google Scholar]
- 30.Chen JY, Wu H, Li H, Hu SL, Dai MM, Chen J. Anti-inflammatory effects and pharmacokinetics study of geniposide on rats with adjuvant arthritis. Int Immunopharmacol. 2015;24:102–109. doi: 10.1016/j.intimp.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Bakdash G, Vogelpoel LT, van Capel TM, Kapsenberg ML, de Jong EC. Retinoic acid primes human dendritic cells to induce guthoming, IL-10-producing regulatory T cells. Mucosal Immunol. 2015;8:265–278. doi: 10.1038/mi.2014.64. [DOI] [PubMed] [Google Scholar]
- 32.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 33.Gorham JD, Güler ML, Fenoglio D, Gubler U, Murphy KM. Low dose TGF-beta attenuates IL-12 responsiveness in murine Th cells. J Immunol. 1998;161:1664–1670. [PubMed] [Google Scholar]
- 34.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through tolllike receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56:2192–2201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 36.Lacerte P, Brunet A, Egarnes B, Duchêne B, Brown JP, Gosselin J. Overexpression of TLR2 and TLR9 on monocyte subsets of active rheumatoid arthritis patients contributes to enhance responsiveness to TLR agonists. Arthritis Res Ther. 2016;18:10. doi: 10.1186/s13075-015-0901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyeko GW, Hatterer E, Herren S, Di Ceglie I, van Lent PL, Reith W, Kosco-Vilbois M, Ferlin W, Shang L. Spatiotemporal expression of endogenous TLR4 ligands leads to inflammation and bone erosion in mouse collagen-induced arthritis. Eur J Immunol. 2016;46:2629–2638. doi: 10.1002/eji.201646453. [DOI] [PubMed] [Google Scholar]
- 38.Elshabrawy HA, Essani AE, Szekanecz Z, Fox DA, Shahrara S. TLRs, future potential therapeutic targets for RA. Autoimmun Rev. 2017;16:103–113. doi: 10.1016/j.autrev.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joosten LA, Abdollahi-Roodsaz S, Dinarello CA, O’Neill L, Netea MG. Toll-like receptors and chronic inflammation in rheumatic diseases: new developments. Nat Rev Rheumatol. 2016;12:344–357. doi: 10.1038/nrrheum.2016.61. [DOI] [PubMed] [Google Scholar]
- 40.Hu F, Li Y, Zheng L, Shi L, Liu H, Zhang X, Zhu H, Tang S, Zhu L, Xu L, Yang Y, Li Z. Toll-like receptors expressed by synovial fibroblasts Perpetuate Th1 and Th17 Cell responses in rheumatoid arthritis. PLoS One. 2014;9:e100266. doi: 10.1371/journal.pone.0100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erridge C, Kennedy S, Spickett CM, Webb DJ. Oxidized Phospholipid inhibition of Toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4. J Biol Chem. 2008;283:24748–24759. doi: 10.1074/jbc.M800352200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen JQ, Szodoray P, Zeher M. Toll-like receptor pathways in autoimmune diseases. Clin Rev Allergy Immunol. 2016;50:1–17. doi: 10.1007/s12016-015-8473-z. [DOI] [PubMed] [Google Scholar]
- 44.Sacre SM, Andreakos E, Kiriakidis S, Amjadi P, Lundberg A, Giddins G, Feldmann M, Brennan F, Foxwell BM. The Toll-like receptor adaptor proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol. 2007;170:518–525. doi: 10.2353/ajpath.2007.060657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakamachi Y, Ohnuma K, Uto K, Noguchi Y, Saegusa J, Kawano S. MicroRNA-124 inhibits the progression of adjuvant-induced arthritis in rats. Ann Rheum Dis. 2016;75:601–608. doi: 10.1136/annrheumdis-2014-206417. [DOI] [PubMed] [Google Scholar]
- 46.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 47.Zvaifler NJ, Firestein GS. Pannus and pannocytes. Alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37:783–789. doi: 10.1002/art.1780370601. [DOI] [PubMed] [Google Scholar]


