Figure 10.
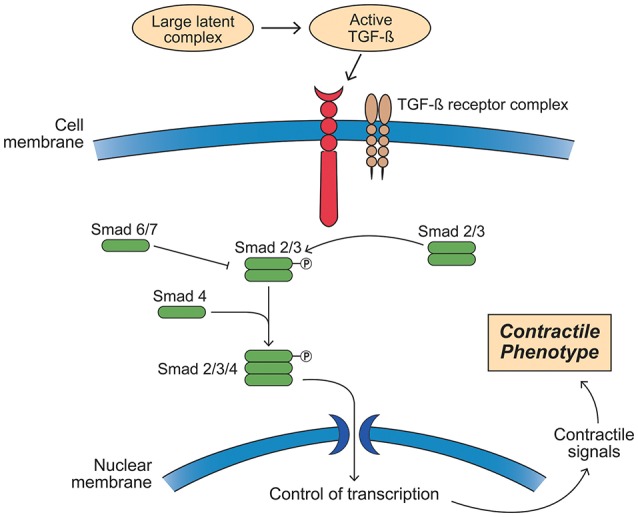
Activation of the transforming growth factor-β (TGF-β) signaling pathway leading to a smooth muscle cell contractile phenotype. Members of the TGF-β superfamily that include TGF-βs, bone morphogenetic proteins (BMPs), and growth and differentiation factors (GDFs) have similar functional properties regulating cell growth, differentiation, apoptosis, and extracellular matrix synthesis in vascular smooth muscle cells (SMCs). TGF-β ligands are synthesized as latent precursor molecules (LTGF-β), which are activated via proteolytic cleavage. Active TGF-β signaling is transmitted through two types of transmembrane serine/threonine protein kinase receptors: TGF-β type I (TGFβRI) and principally type II (TGFβRII) and mediated by a sequence of phosphorylated Smad proteins. In addition to the canonical Smad signaling pathway that directly regulates the transcription of Smad-dependent target genes, TGF-β function can also be mediated by Smad-independent pathways including MAPK signaling pathways, such as p38 MAPK and c-Jun NH2-terminal kinase, phosphatidylinositol 3-kinase/Akt pathway, and Wnt signaling. TGF-β signaling via TGFβRII plays a pivotal role in both second heart field and cardiac neural crest derived SMC phenotype differentiation during vascular development as well as SMC phenotypic switching in disease states. TGF-β signaling induces SMCs to change shape into elongated SMC shape accompanied by an up-regulation of SMC contractile proteins.
