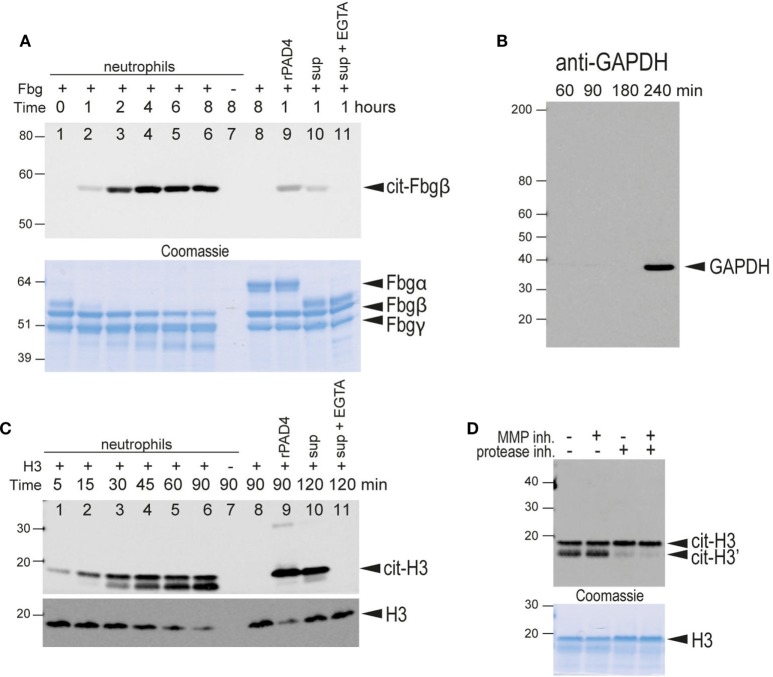
Figure 5.
Citrullination of extracellular substrates by intact neutrophils or neutrophil-conditioned media. (A) Upper panel, anti-citrullinated fibrinogen immunoblot of the supernatant from the incubation of fibrinogen for the indicated times in the presence of human neutrophils (lanes 1–7). Lane 7, fibrinogen was omitted. Lane 8 only contained fibrinogen. Lane 9, fibrinogen and recombinant PAD4. Lane 10, neutrophil-conditioned media. Lane 11, neutrophil with 2 mM EGTA. Lower panel, Coomassie Brilliant blue staining as a loading control. The bands corresponding to the α, β, and γ chains of fibrinogen are indicated. Note that α-fibrinogen is rapidly degraded by neutrophil-associated proteases. (B) Control immunoblot with anti-GAPDH, an intracellular protein, to demonstrate that significant lysis of neutrophils occurs only after 4 h of incubation with Triton X at 37°C under the experimental conditions. (C) Upper panel, anti-citrullinated histone H3 immunoblot of the supernatant from the incubation of histone H3 for the indicated time periods in the presence of human neutrophils (lanes 1–7). Lane 7, histone was omitted. Lane 8 only contained histone H3. Lane 9 contained histone H3 and recombinant PAD4. Lane 10, neutrophil-conditioned media. Lane 11, neutrophil with 2 mM EGTA. Note that citrullinated histone H3 is also cleaved by neutrophil-associated protease(s) to generate a slightly smaller protein. Lower panel, anti-histone H3 immunoblot as a loading control. This antibody does not recognize the proteolytically cleaved H3. (D) A similar experiment performed in the presence of an MMP inhibitor, a protease inhibitor cocktail, or both, as indicated. Lower panel, Coomassie Brilliant blue staining as a loading control. All data are representative of five independent experiments with different donors.