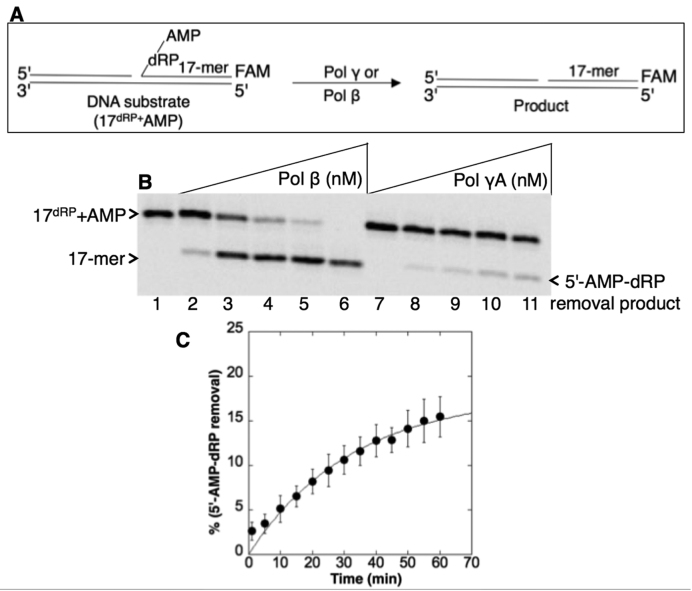
Figure 2.
Purified pol γ lyase activity on the 5′-AMP-dRP-including BER intermediate. (A) Illustrations of the single-nucleotide gapped DNA substrate with 5′-AMP-dRP (17dRP+AMP) and the dRP lyase reaction product (17-mer). (B) Lane 1 is the minus enzyme control. Lanes 2–6 are the positive control reaction products of pol β (20–500 nM), and lanes 7–11 are the reaction products of pol γ (20–500 nM) for removal of the 5′-AMP-dRP group. (C) Rate of 5′-AMP-dRP removal by pol γ. The dRP lyase assay was performed under single-turnover conditions with purified pol γ (500 nM) in excess over the DNA substrate (100 nM). A plot of product formation was obtained by fitting the data to an exponential time course, yielding the rate of pol γ as kobs of 0.03 min−1. The data represent the average of three independent experiments ± SD.