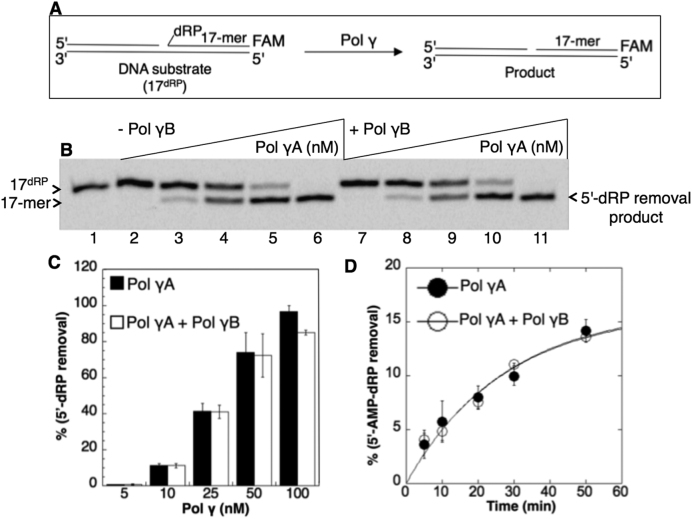
Figure 3.
Lack of influence of the pol γB subunit on the pol γA dRP lyase activity. (A) Illustrations of the single-nucleotide gapped DNA substrate (17dRP) and the dRP lyase reaction product (17-mer). (B) Lane 1 is the minus enzyme control. Lanes 2–6 are the reaction products of 5′-dRP removal by pol γA alone (5–100 nM). Lanes 7–11 are the reaction products of 5′-dRP removal by the holoenzyme including pol γA (5–100 nM) plus pol γB (20–400 nM). (C) The graph shows pol γ concentration-dependent changes in the products of 5′-dRP removal in the absence and presence of the pol γB subunit. The data represent the average of three independent experiments ± SD. (D) The rates of the 5′-AMP-dRP removal are 0.036 min−1 (pol γA) and 0.04 min−1 (pol γA+pol γB). The lyase assays were performed in the absence and presence of pol γ accessory subunit (pol γB) under same single-turnover conditions. The data represent the average of three independent experiments ± SD.