Fig. 2.
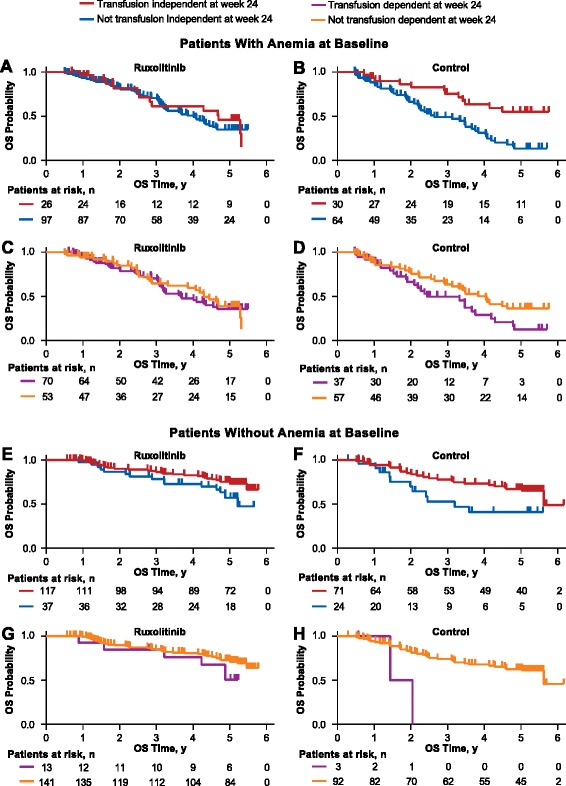
Overall survival: 5-year pooled data stratified by baseline anemia status and week 24 transfusion status. OS analysis of 5-year pooled data from the COMFORT-I and -II trials stratified by baseline anemia status and week 24 transfusion status. Patients in the ruxolitinib and control groups were stratified by anemia status at baseline and (a, b, e, f) transfusion independence status at week 24 or (c, d, g, h) transfusion dependence status at week 24. OS probability in the ruxolitinib group was not significantly affected by transfusion status at week 24 (transfusion independent vs not independent, P = 0.1322*; transfusion dependent vs not dependent, P = 0.4547*), but was significantly affected in the control group (transfusion independent vs not independent, P = 0.0004*; transfusion dependent vs not dependent, P = 0.0323*). Baseline anemia was defined as receiving any units of RBCs within 12 weeks before baseline measurement or having baseline hemoglobin < 10 g/dL; nonanemic was defined as not meeting criteria for anemia. Transfusion independence at week 24 was defined as the absence of RBC transfusions and hemoglobin levels ≥ 8 g/dL during weeks 13 to 24; not transfusion independent at week 24 was defined as requiring RBC transfusions or hemoglobin levels < 8 g/dL during weeks 13 to 24. Transfusion dependence at week 24 was defined as requiring ≥ 4 units of RBCs or hemoglobin levels < 8 g/dL during weeks 17 to 24; not transfusion dependent at week 24 was defined as requiring < 4 units of RBCs and hemoglobin levels ≥ 8 g/dL during weeks 17 to 24. Originally presented at the American Society of Hematology 58th Annual Meeting [13]. IPSS, International Prognostic Scoring System; OS, overall survival; RBC, red blood cell. *Stratified by study, IPSS risk, and baseline anemia status
