Abstract
Fruits rich in polyphenols, such as pomegranates, have been shown to have health benefits relating to their antioxidant and anti-inflammatory properties. Using data obtained from PubMed and Scopus, this article provides a brief overview of the therapeutic effects of pomegranate on chronic inflammatory diseases (CID) such as inflammatory bowel disease (IBD), rheumatoid arthritis (RA), metabolic and cardiovascular disorders, and other inflammatory-associated conditions, with an emphasis on fruit-derived juices. Most studies regarding the effects of pomegranate juice have focused on its ability to treat prostate cancer, diabetes, and atherosclerosis. However, pomegranate juice has shown therapeutic potential for many other illnesses. For instance, a small number of human clinical trials have highlighted the positive effects of pomegranate juice and extract consumption on cardiovascular health. The beneficial effects of pomegranate components have also been observed in animal models for respiratory diseases, RA, neurodegenerative disease, and hyperlipidaemia. Furthermore, there exists strong evidence from rodent models suggesting that pomegranate juice can be used to effectively treat IBD, and as an anti-inflammatory agent to treat CID. The effects of pomegranate intake should be further investigated by conducting larger and more well-defined human trials.
Keywords: pomegranate, Punica granatum, pomegranate juice, ellagitannins, inflammation, inflammatory diseases, anti-inflammatory properties
1. Introduction
Inflammation is a complex biological response to tissue injury and infection. Chronic inflammation has been shown to be involved in the onset and development of a range of disorders. Chronic inflammatory disease (CID) is a general term used for conditions where persistent inflammation plays a central role in disease pathology [1]. Examples of CID include rheumatoid arthritis (RA), inflammatory bowel disease (IBD), chronic obstructive pulmonary disease (COPD), asthma, and psoriasis. Patients with CID present with heavy infiltration of inflammatory cells at the site of disease—e.g., joints, intestinal mucosa, lungs, and skin—and show elevated levels of inflammatory mediators [2]. Dysfunctional inflammatory responses have also been implicated as contributors to other chronic diseases such as atherosclerosis, type 2 diabetes, obesity, insulin resistance, and certain neurodegenerative diseases (e.g., Alzheimer’s disease) [3].
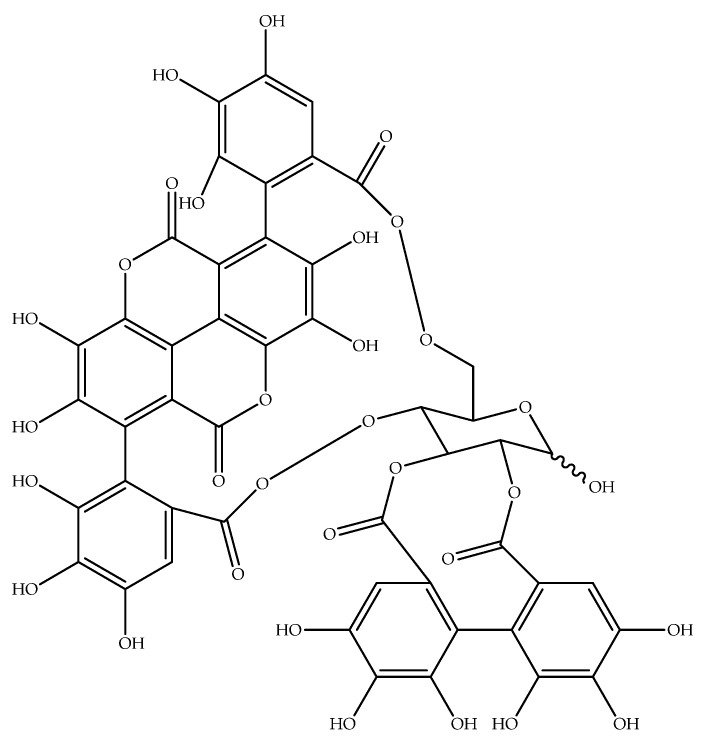
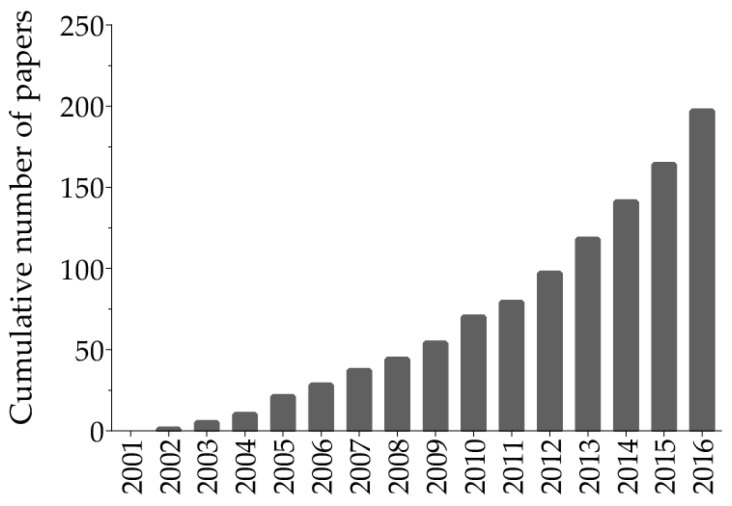
Ellagitannins (ET) and ellagic acid (EA) are polyphenols present in some fruits, nuts, and seeds—such as pomegranates, black raspberries, raspberries, strawberries, walnuts, and almonds [4]. It has been shown that ET-rich fruits have anti-oxidant, anti-inflammatory, anti-neoplastic, and chemo-preventive properties [5]. Pomegranate (Punica granatum L.) is a rich source of ET punicalagin (Figure 1), which aroused considerable interest in pomegranate fruit as a novel therapeutic within the last several years (Figure 2).
Figure 1.
The structure of punicalagin, the main polyphenolic compound present in pomegranate.
Figure 2.
The popularity of health benefits of ellagitannins over time as shown by the cumulative number of papers from 2001 to 2016 obtained by a basic search on the PubMed database using “ellagitannins” and “health” as terms search.
Although it is widely accepted that pomegranate intake can provide significant health benefits, the results of human clinical trials using pomegranate juice as a therapeutic agent have been inconsistent. This may, in part, be due to variability in the composition of the administered pomegranate products. Several studies have suggested that pomegranate intake has positive effects on blood pressure [6,7] and cardiovascular risk in diabetic [8,9], obese [10,11], hypertensive and ischemic patients [12,13]. Conversely, a meta-analysis found that pomegranate had no effect on lipid profiles [14]. In addition, a meta-analysis of data from five prospective trials did not find a significant effect of pomegranate juice on plasma C-reactive protein (CRP) levels [15].
No clear consensus has yet emerged on the putative anti-inflammatory effects of pomegranate intake on CID. Therefore, we conducted a systematic review to provide an overview of the evidence of the potential benefits of pomegranate products—with an emphasis on fruit-derived juices—on this occurrence.
2. Search Strategy
We performed an extensive search using the PubMed and Scopus databases in April 2017. The following keywords and Medical Subject Headings (MeSH) terms were combined: “pomegranate” or “Punica granatum”, “inflam*”, and “disease*”. We did not use language restrictions, and reviews were excluded. The search strategies were as follows: Medline search strategy (pomegranate*) OR (Punica granatum) AND inflam* AND disease* NOT review*; Scopus search strategy (TITLE-ABS-KEY (pomegranate*) OR TITLE-ABS-KEY (Punica granatum) AND TITLE-ABS-KEY (inflam*) AND TITLE-ABS-KEY (disease*)) AND (LIMIT-TO (DOCTYPE, “ar”)).
3. Results and Discussion
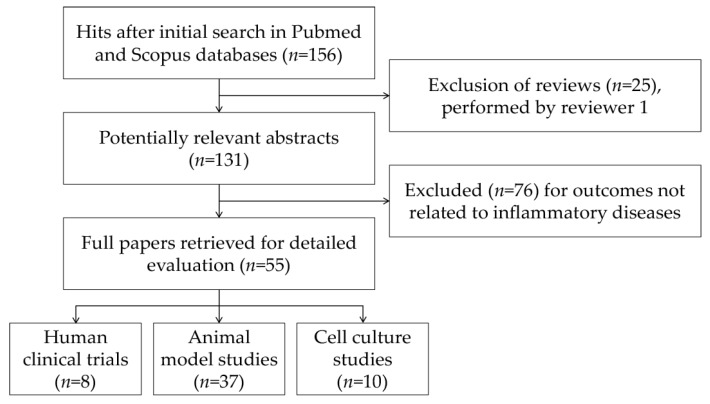
The literature selection process was conducted following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) recommendations [16]. The initial search yielded 156 hits after the exclusion of duplicates. During the screening process (reviewing of titles and abstracts), 25 records were excluded. After full-text analysis, another 76 papers were excluded. Altogether, 55 papers were selected for detailed evaluation. Both reviewers independently selected the evaluated articles. Figure 3 shows the flow chart of the selection procedure of the papers.
Figure 3.
Flow chart of papers included in the review.
Human clinical trials are relatively few (Table 1) and were mainly focused on the effects of pomegranate consumption on cardiovascular health.
Table 1.
Published research studies on the potential beneficial effects on Chronic Inflammatory Disease (CID) of pomegranate products.
| CID | Human Clinical Trials (No. of Subjects) | Animal Model Studies | Cell Culture Studies |
|---|---|---|---|
| Asthma and COPD | - | 3 [17,18,19] | - |
| IBD | - | 8 [20,21,22,23,24,25,26,27] | 2 [28,29] |
| Immune system | - | - | 2 [30,31] |
| Metabolic and cardiovascular disorders § | 7 (51 [32], 13 [33], 31 [34], 30 [35], 101 [36], 24 [37], 27 [38]) | 14 [39,40,41,42,43,44,45,46,47,48,49,50,51,52] | - |
| Neurodegenerative diseases | - | 8 [53,54,55,56,57,58,59,60] | 4 [53,61,62,63] |
| Psoriasis | - | - | - |
| RA | 1 (55 [64]) | 1 [65] | 1 [66] |
| Other disorders §§ | - | 3 [67,68,69] | 1 [70] |
COPD: chronic obstructive pulmonary disease; IBD: inflammatory bowel disease; RA: rheumatoid arthritis. § atherosclerosis, type 2 diabetes, obesity, metabolic syndrome, insulin resistance, hyperlipidaemia. §§ cell proliferation, hyperplasia, metaplasia, cancer.
3.1. Findings Related to Pomegranate Products Consumption and CID in Humans
Table 2 summarises the data published on human trials related to pomegranate intake. Unfortunately, there exists a limited number of human trials available concerning the effects of pomegranate on the outcomes of patients with CID. Within these studies, we found the tested dietary products and experimental designs to be highly variable. Only one trial has been conducted using pomegranate seed oil [32], whereas most other studies have been carried out using either pomegranate juice [33,34,35,36,37] or the commercially available whole fruit phenolic extract POMxTM [37,38,64], which are both safe and well-tolerated by CID patients. The doses of pomegranate juice or extract used also varied from study to study as groups used a range of 100 to 500 mL [35] of juice and 0.5 to 1 g of POMxTM [37,38]. Additionally, the study designs used differ for each trial—some used pre- and post-test schemes [33,34] or randomised placebo-controlled tests [32,35,36,38,64], and one trial even lacked an appropriate placebo comparator [37]. Finally, the duration of each study is not consistent—some performed acute (1 day) [33] or short-term (1 week) [35] trials and others assessed the long-term (6–12 months) effects of pomegranate intake [36,38]. While these studies focused primarily on patients affected by metabolic and cardiovascular disorders [32,33,34,35,36,37,38] and RA [64], there exist three clinical trials that are currently in progress exploring the therapeutic potential of pomegranate juice on IBD, memory impairment, ageing and skin inflammation (Table 3).
Table 2.
Summary of findings related to pomegranate products consumption and CID in humans.
| Study Design | Population | Subjects (Gender, No., Age) | Intervention | Control/Comparator | Duration | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| Double-blind, placebo-controlled, randomised | Dyslipidaemic patients | F & M: 51, 42–64 years | Pomegranate seed oil 400 mg/day × 2 | Paraffin 400 mg/day × 2 | 4 weeks | ↓ TG, ↓ HDL-C, ↓ TG/HDL-C ratio, ↔ TNF-α | Asghari et al., 2012 [32] |
| Pre- and post-test | Hypertensive patients | M: 13, 39–68 years | Pomegranate juice 150 mL/day | - | 1 day | ↓ SBP, ↓ DBP, ↔ CRP, ↔ ICAM-1, ↔ VCAM-1, ↔ IL-6, ↔ E-selectin | Asgary et al., 2013 [33] |
| Pre- and post-test | Type 2 diabetic patients | F: 16, M: 15, 38–54 years | Concentrated pomegranate juice 50 g/day | - | 4 weeks | ↔ SBP, ↔ DBP, ↑ TC, ↑ HDL-C, ↔ TG, ↔ LDL-C, ↔ glycaemia, ↓ IL-6, ↔ TNF-α, ↔ CRP, ↓ adiponectin, ↑ TAC | Shishehbor et al., 2016 [34] |
| Double-blind, placebo-controlled, randomised crossover | Patients with metabolic syndrome | F: 16, M: 14, 42–62 years | Pomegranate juice 500 mL/day | Placebo 500 mL/day | 1 week | ↓ SBP, ↓ DBP, ↓ CRP, ↑ TG, ↑ VLDL-C | Moazzen & Alizadeh 2017 [35] |
| Double-blind, placebo-controlled, randomised | Haemodialysis patients | F: 46, M: 55, 55–81 years | Pomegranate juice 100 mL/day | Placebo 100 mL/day × 1 | 12 months | ↓ IL-6, ↓ TNF-α, ↓ MPO, ↓ AOPP, ↓ oxidised fibrinogen, ↓ MDA | Shema-Didi et al., 2012 [36] |
| Pilot, open, randomised crossover | Haemodialysis patients | F: 13, M: 11, 47–75 years | Pomegranate juice 100 mL/day; Pomegranate extract POMxTM 1050 mg/day (both containing 650 mg GAE) | - | 4 weeks | ↔ SBP, ↔ DBP, ↔ CRP, ↔ IL-6, ↔ F2-isoprostanes, ↔ isofurans, TG, ↔ TC, ↔ HDL-C, ↔ LDL-C | Rivara et al., 2015 [37] |
| Double-blind, placebo-controlled, randomised, parallel-arm | Haemodialysis patients | F: 10, M: 17, 49–59 years | Pomegranate extract POMxTM 1 g/day (containing 600–755 mg GAE) | Placebo 1 pill/day | 6 months | ↓ SBP, ↓ DBP, ↔ CRP, ↔ IL-6, ↔ TC, ↔ HDL-C, ↔ LDL-C, ↔ TG, ↔ ORAC, ↔ AOPP, ↔ 8-OHdG, ↔ ox-LDL, ↔ arylesterase activity, ↔ lactonase activity, ↔ PON activity | Wu et al., 2015 [38] |
| Double-blind, placebo-controlled, randomised | RA patients | F & M: 55, 37–61 years | Pomegranate extract POMxTM 250 mg/day × 2 | Placebo (cellulose) 250 mg/day × 2 | 8 weeks | ↔ CRP, ↔ MMP3, ↔ MDA, ↑ GPx, ↓ ESR, ↓ DAS28, ↓ HAQ, ↓ swollen joints, ↓ tender joints, ↓ pain intensity, ↓ morning stiffness | Ghavipour et al., 2017 [64] |
↑: increase; ↔ : no change; ↓: decrease; 8-OHdG: 8-hydroxy-20-deoxyguanosine; AOPP: advanced oxidation protein products; CRP: C-reactive protein; DAS28: disease activity score; DBP: diastolic blood pressure; ESR: erythrocyte sedimentation rate; F: female; GAE: gallic acid equivalents; GPx: glutathione peroxidase; HAQ: health assessment questionnaire; HDL-C: high-density lipoprotein–cholesterol; ICAM-1: intracellular adhesion molecule-1; IL-6: interleukin 6; LDL-C: low-density lipoprotein–cholesterol; M: male; MDA: malondialdehyde; MMP3: matrix metalloproteinase-3; MPO: myeloperoxidase; ORAC: oxygen radical absorbance capacity; ox-LDL: oxidised low-density lipoprotein; PON: paraoxonases; SBP: systolic blood pressure; TAC: total antioxidant capacity; TC: total cholesterol; TG: triglycerides; TNF-α: tumour necrosis factor α; VCAM-1: vascular cell adhesion molecule-1; VLDL-C: very low-density lipoprotein–cholesterol.
Table 3.
Ongoing clinical trials on pomegranate juice [72].
| clintrials.gov Identifier | Study Focus | Study Design, Duration | Sponsor | Estimated Enrolment | Study Start Date | Estimated Completion Date |
|---|---|---|---|---|---|---|
| NCT02093130 | Memory in older adults | Double-blind, placebo-controlled, parallel arm, randomised, 12 months | University of California (Los Angeles, CA, USA) | 212 | January 2014 | December 2017 |
| NCT02258776 | Ageing and inflammation of the skin | Single-blind, placebo-controlled, parallel arm, randomised, 12 weeks | University of California (Los Angeles, CA, USA) | 15 | October 2015 | January 2018 |
| NCT03000101 | Inflammation in IBD | Double-blind, placebo-controlled, parallel arm, randomised, 12 weeks | St. Orsola-Malpighi Hospital (Bologna, Italy) | 36 | December 2016 | June 2018 |
Even though the use of differing approaches renders it difficult to draw general conclusions, there is a generally positive effect of pomegranate consumption in patients with chronic inflammatory disorders. Pomegranate juice appears to have promising hypotensive properties in patients with hypertension [33] or metabolic syndrome [35,71], and in patients undergoing dialysis [38]. It also resulted in a slight amelioration of lipid profiles in patients with cardiovascular disease (CVD), as pomegranate intake elevated endogenous levels of high-density lipoprotein (HDL)-cholesterol [34] and reduced triglyceride (TG) levels [32]. However, several studies have been unable to confirm pomegranate’s TG- and cholesterol-lowering effect [34,35,37,38]. With regards to risk factors for CVD, the consumption of 150 mL of pomegranate juice—restricted to one serving per day—did not influence the level of circulating soluble adhesion molecules or markers of atherosclerosis and subclinical coronary heart disease (CHD) in hypertensive individuals [33]. Evidence for the beneficial effects of pomegranate on CVD was obtained using long-term consumption (100 mL daily for one year) [36] or with a greater intake of juice (500 mL daily) [35].
Markers of systemic inflammation have not been consistently evaluated in many of these studies, a factor which has an impact on the conclusions drawn from each trial. It has been shown that circulating pro-inflammatory cytokines in patients affected by cardiovascular disorders are slightly reduced by the consumption of pomegranate juice and extract. Notably, a decrease in the level of interleukin 6 (IL-6)—a well-known pro-inflammatory cytokine—has been demonstrated in patients with type 2 diabetes [34] and in patients undergoing haemodialysis [36]. Conversely, pomegranate intake has no effect on the plasma levels of CRP or tumour necrosis factor α (TNF-α), except in patients with metabolic syndrome [35] or those undergoing dialysis [36]. It is generally accepted that the anti-inflammatory effects of pomegranate intake are mediated by its anti-oxidant properties. In agreement, pomegranate intake results in an improvement in plasma antioxidant capacity, as it has been shown to decrease the prevalence of oxidatively damaged molecules and increase anti-oxidant-dependent immune responses in patients affected by CID [34,64].
In conclusion, evidence from human trials indicates that pomegranate, when administered as a juice in high doses or for an extended period, can reduce oxidative stress and systemic inflammation. However, there exist limitations to many of these studies as these trials were limited in sample size, used short durations of supplementation, and/or lacked necessary controls.
3.2. Evidence of Anti-Inflammatory Effects of Pomegranate or Pomegranate-Derived Products in Different Animal Models of CID
Animal models have been used to investigate the pathology of a wide range of chronic inflammatory diseases—COPD, IBD, metabolic and cardiovascular disorders, neurodegenerative diseases, RA, cancer—in which inflammation is induced experimentally by genetic manipulation or pharmacologically (diet, drugs, or exogenous toxicants) (Table 4). These studies examined the effects of different pomegranate-related products, including whole fruit juice [19,21,26,41,42,43,48,50,60] or extract [22,24,26,40,43,44,52,55,56,59,67,68,69], extracts obtained using various parts of the fruit (e.g., peel [17,45,47,58], seeds [20,43,46,50,54], flowers [27,45], leaves [18]), concentrated extracts (POMxTM [65], Pomanox® [51]) and bioactive molecules found in pomegranates (punicalagin [53], EA [23,24,25,49,57], punicic acid [20]) and their derived metabolites (urolithins [22]). The administered doses of these extracts varied in range between 10 to 80 mg/kg [24,25,57]. Based on the previous findings of Kaulmann and Bohn (2016) [73], the doses administered in these in vivo studies probably resulted in supra-physiological serum concentrations of pomegranate metabolites obtained only through supplements in humans.
Table 4.
Overview of the anti-inflammatory effects of pomegranate or pomegranate-derived products in animal models of CID.
| Disease Model | Animal Model | Tested Product(s), Vehicle, Duration | Disease Induction | Effects | Reference |
|---|---|---|---|---|---|
| Respiratory diseases | BalbC mice | Pomegranate peel aqueous extract (200 mg/kg b.w.) via intraperitoneal injection for 2 days | LPS-induced lung inflammation | ↓ total cells in BAL, ↓ neutrophils in BAL | Bachoual et al., 2011 [17] |
| BalbC mice | Encapsulated pomegranate leave extract (10 mg/mL) or non-encapsulated pomegranate leave extract (20 mg/kg b.w.) via nostril for 4 days | Ovalbumin-induced asthma | ↓ leukocytes, neutrophils, and eosinophils in BAL, ↓ macrophages in BAL (non-encapsulated extract only), ↔ lymphocytes in BAL, ↓ IL-1β and IL-5 in BAL | de Oliveira et al., 2013 [18] | |
| C57BL/6J mice | Pomegranate juice (80 μmol/kg b.w.) via bottle for 1 week or 1 month or 3 months | Cigarette smoke-induced lung stress | ↓ IL-1β and IL-6 expression in lung (1 week only), ↓ TNF-α expression in lung | Husari et al., 2016 [19] | |
| IBD | Wistar rats | Punicic acid (400 mg/0.5 mL PBS) or pomegranate seed oil (0.5 mL) via oral administration for 10 days | TNBS-induced colitis | Punicic acid: ↓ Wallace and Ameho scores, ↓ MPO activity in colon, ↓ F2-isoprostane in colon; Pomegranate seed oil: ↓ Wallace and Ameho scores | Boussetta et al., 2009 [20] |
| Swiss albino mice | Pomegranate flower hydro-alcoholic extract (100 or 200 mg/kg b.w.) or EA-rich fraction of pomegranate flower (100 or 200 mg/kg b.w.) via oral administration for 7 days | DSS-induced colitis | ↓ macroscopic and histopathological changes in colon, ↓ colon MPO activity, ↓ histamine content in colon, ↓ MDA level in colon, ↓ superoxide anion production in colon | Singh et al., 2009 [27] | |
| Fischer rats | Pomegranate extract (250 mg/kg b.w.) or urolithin A (15 mg/kg b.w.) via chow for 10 days | DSS-induced colitis | ↓ colon tissue damage (urolithin A only), ↑ FRAP (pomegranate extract only), ↓ MDA in colon (pomegranate extract only), ↓ COX-2 gene and protein expression in colon, ↓ iNOS expression in colon, ↓ PGE2 and NO levels in colon (pomegranate extract only), ↓ PTGES protein expression in colon | Larrosa et al., 2010 [22] | |
| Wistar rats | EA (10 and 20 mg/kg b.w.) via oral gavage for 48, 24 and 1 h prior to the induction of colitis and 24 h later | TNBS-induced colitis | ↓ colon macroscopic damage, ↓ b.w. loss, ↓ colon weight/length, ↓ histological damage in colon, ↓ colon MPO activity, ↓ iNOS and COX-2 protein expression in colon, ↓ JNK and ERK phosphorylation in colon, ↓ NF-κB activation in colon | Rosillo et al., 2011 [25] | |
| Wistar rats | Pomegranate extract (250 or 500 mg/kg feed) or EA (10 mg/kg feed) or EA-enriched pomegranate extract (pomegranate extract 250 mg/kg feed + EA 10 mg/kg feed) via chow for 30 days prior to the induction of colitis and 14 days later | TNBS-induced colitis | ↓ colon macroscopic damage, ↓ b.w. loss (all treatments, apart from extract 250 mg/kg), ↓ colon weight/length (EA and EA-enriched extract only), ↓ colon MPO activity, ↓ TNF-α level in colon, ↓ iNOS and COX-2 protein expression in colon, ↓ JNK and ERK phosphorylation in colon, ↓ NF-κB activation in colon, ↔ colon PPAR-γ protein expression | Rosillo et al., 2012 [24] | |
| C57BL/6 mice | EA (0.5% w/w, equivalent to 25 mg/mouse) via chow for 56 days | DSS-induced colitis | ↓ disease symptoms, ↓ DAI, ↓ iNOS and COX-2 protein expression in colon, ↓ JNK and ERK phosphorylation in colon, ↓ NF-κB activation in colon, ↓ IL-6 gene expression in colon, ↓ STAT3 phosphorylation in colon | Marín et al., 2013 [23] | |
| Sprague-Dawley rats | Pomegranate beverage (containing 2504.74 mg/L GAE) ad libitum for 3 weeks prior to the induction of colitis and 7 weeks later | DSS-induced colitis | ↓ colonocyte proliferative index, ↓ expression of hs-CRP, TNF-α, IL-1β, and IL-6 in intestinal mucosa, ↓ IL-1β and IL-6 levels in serum, ↑ IL-10 level in serum, ↓ p-p70-S6K/p70-S6K, ↓ p-rpS6/rpS6 | Kim et al., 2016 [21] | |
| Sprague-Dawley rats | Pomegranate juice (400 mg/kg b.w.) or pomegranate powder (4 mg/kg b.w.) via oral administration for 18 days | DNBS-induced colitis | ↔ histopathological scores, ↓ CMDI and DAI, ↓ MDA in colon (juice only), ↔ colon MPO activity, ↓ colon NO production, ↓ colon SOD activity, ↓ serum cortisol level, ↓ IL-1β, IL-18, TNF-α, and NF-κB expression in colon | Shah et al., 2016 [26] | |
| Metabolic and cardiovascular disorders | Zucker rats | Concentrated pomegranate juice or pomegranate fruit extract (6.25 mL/L) via drinking water or pomegranate seed oil (1 mL/L) via chow for 5 weeks | Obese metabolic syndrome model | ↔ TC, ↔ LDL-C, ↔ HDL-C, ↑ TG (seed oil only), ↔ daytime MAP, ↔ BPM, ↔ motor activity, ↓ arterial TSP-1 protein expression, ↑ eNOS protein expression (apart from oil), ↓ arterial TGF-β1 protein expression (except oil), ↓ nitrate and nitrite levels (apart from oil), ↔ insulin and glucose levels | de Nigris et al., 2007 [43] |
| db/db mice | Pomegranate seed oil (1 g/100 g feed) via chow for 30 days | Diabetes and obesity model | ↓ glycaemia, ↓ blood insulin, ↑ expression of genes PPAR-α, CD36, and FABP4 in adipose tissue, ↔ expression of genes PPAR-γ, ACAD, and SCD1 in adipose tissue, ↑ expression of genes PPAR-γ, CD36, FABP4, ACAD, and SCD1 in muscle, ↔ expression of genes PPAR-α in muscle, ↓ TNF-α expression and NF-κB activation in adipose tissue and liver | Hontecillas et al., 2009 [46] | |
| CD-1 mice | Pomegranate juice (12.5 mL/L juice diluted in water, equivalent to 0.35 mmol polyphenols) via drinking water for 4 months | Streptozotocin-induced diabetes | ↑ hepatic PON-1 expression and activity, ↓ glycaemia, ↔ blood TC and TG levels | Betanzos-Cabrera et al., 2011 [41] | |
| Sprague-Dawley rats | Pomegranate juice (100 μL) via gastric gavage for 10 weeks | Streptozotocin-induced diabetes | ↔ GSH in lung, ↑ SOD activity in lung, ↓ protein carbonyl content in lung, ↓ serum sialic acid, ↓ eNOS protein in lung | Çukurova et al., 2012 [42] | |
| SR-BI/apoE double knockout mice | Pomegranate extract (307.5 ml/L) via drinking water for 2 weeks | Coronary heart disease model | ↑ TC, ↔ serum apoA and apoB, ↓ atherosclerosis, ↔ SAA and serum MCP-1, ↓ MCP-1 in plaques, ↓ lipid accumulation, macrophage infiltration, and MCP-1 levels in heart, ↓ myocardial fibrosis, cardiac enlargement, and ECG abnormalities | Al-Jarallah et al., 2013 [40] | |
| BalbC mice | Pomegranate peel extract (0.2% w/v diluted in water, equivalent to 6 mg per mouse) via drinking water for 4 weeks | High-fat diet-induced obesity and hypercholesterolaemia | ↔ body weight gain, ↔ adiposity, ↔ glycaemia and insulin response, ↓ serum TC and LDL-C, ↔ serum HDL-C and TG, ↔ hepatic TC and TG, ↔ IL-1β, IL-6, and COX-2 expression in liver, ↔ IL-1β expression in colon, ↓ IL-6 and COX-2 expression in colon | Neyrinck et al., 2013 [47] | |
| Wistar rats | EA (0.8 g/kg feed) via chow for 8 weeks after the induction of metabolic syndrome | High-fat and high-carbohydrate diet-induced metabolic syndrome | ↑ retroperitoneal, epididymal, omental, and total abdominal fat, ↔ whole-body fat mass, ↓ whole-body lean mass, ↓ glycaemia, ↓ plasma TG, TC, NEFA, uric acid, urea, and CRP, ↓ plasma ALT, AST, ALP, and LDH activity, ↔ plasma albumin and bilirubin, ↓ SBD, ↑ coronary endothelial-dependent relaxation, ↔ Nrf2 protein expression in heart, ↑ Nrf2 protein expression in liver, ↓ NF-κB expression in heart and liver, ↑ CPT1 expression in heart and liver | Panchal et al., 2013 [49] | |
| Wistar Albino Glaxo rats | Pomegranate extract (300 mg/kg b.w.) via chow for 8 weeks | High-fat diet-induced metabolic syndrome | ↔ weight of epididymal adipose tissue, ↔ glycaemia, ↓ LDL-C, ↔ TC, HDL-C, TG, and FFA, ↔ SBP, ↓ serum corticosterone, ↔ adrenal corticosterone, ↓ serum IL-6 and TNF-α, ↓ TG in liver | Dushkin et al., 2014 [44] | |
| Sprague-Dawley rats | PUNI-enriched pomegranate extract (150 mg/kg b.w.) via oral gavage for 8 weeks | High-fat diet-induced NAFLD | ↓ body weight gain, ↓ serum TG, HDL-C, and LDL-C, ↔ serum C, ↓ serum insulin, leptin, and adiponectin, ↓ HOMA-IR, ↓ serum ALT level, ↓ liver tissue weight, ↓ hepatic TG and TC, ↓ expression of SREBP-1c precursor protein, ↔ expression of SREBP-1c mature protein, expression of FA biosynthesis-related genes (↓ SREBP-1c, ↓ FAS, ↓ ACC1, ↓ SCD1), expression of TG biosynthesis-related genes (↓ ACLY, ↔ GPAM, ↑ DGAT-1 and-2), ↓ serum CRP level, IL-1β, IL-4, IL-6, and TNFα, ↓ serum IgA, IgG, and IgM, ↓ protein carbonyl content in liver tissue and liver mitochondria, ↓ lipid peroxidation in liver, ↑ hepatic total SOD activity, ↓ hepatic GSH and GSSG levels, ↑ GSH/GSSG ratio, ↓ Nrf2, HO-1, NQO-1, and UCP2 protein expression in liver, ↑ ATP content in liver, ↑ activities of mitochondrial complexes I, II, and IV in liver, ↑ expression of genes PGC-1-α and PPAR-α in liver, ↔ expression of PGC-1β gene in liver, ↑ PGC-1α protein expression in liver, ↑ expression of genes CPT1A, CPT1B, and ACAD in liver | Zou et al., 2014 [52] | |
| Pigs | Pomegranate extract Pomanox® (625 mg equivalent to 200 mg punicalagins) via chow for 10 days | High-fat diet-induced coronary endothelial dysfunction | ↑ coronary endothelial-dependent relaxation, ↑ Akt and eNOS phosphorylation in coronary artery, ↔ MCP-1 gene expression in coronary artery, ↓ MCP-1 protein content in coronary artery, ↓ coronary DNA oxidative damage, ↓ LDL-C oxidation | Vilahur et al., 2015 [51] | |
| Sprague-Dawley rats | Pomegranate juice concentrate (equivalent to 80 μmol polyphenols/mL) via drinking water for 5 weeks | Cigarette smoking-induced cardiac hypertrophy | ↔ DBP and SBP, ↓ ROS in aortic tissue, ↓ heart to body weight ratio, ↓ fibrotic marker (ObR and Fn1) and kinin receptor (Bdkrb1 and Bdkrb2) expression in aorta, ↓ IL-1β expression in aorta, ↔ TNF-α expression in aorta | Al Hariri et al., 2016 [39] | |
| C57Bl/6 mice | Pomegranate peel (250 mg/kg b.w.) or Pomegranate flower extract (250 mg/kg b.w.) or Pomegranate seed oil (2 mL/kg b.w.) for 6 weeks | High-fat and high-sugar diet-induced obesity | ↔ b.w. gain, ↓ glycaemia (28 days- seed oil treatment only), ↔ plasma insulin level, ↔ plasma TC, HDL-C, and TG, ↔ hepatic ALT and AST, ↔ hepatic TG, ↑ plasma IL-2 (peel extract only), ↓ plasma IL-6 (apart from flower extract), ↑ plasma IL-10 (flower extract only), ↓ plasma TNF-α (apart from peel extract), ↑ IFN-γ (seed oil only) | Harzallah et al., 2016 [45] | |
| Sprague-Dawley rats | Pomegranate juice (60 mL) via drinking water for 7 weeks | High-fat and high-sugar diet-induced NAFLD | ↓ plasma ALT and AST, ↔ plasma GGT and ALP, ↓ glycaemia and insulin, ↓ plasma TG, ↔ plasma TC, HDL-C, and LDL-C, ↓ hepatic IL-1β, IL-6, TNF-α, and TGF-β1 expression, ↑ hepatic IL-10 expression, ↔ GSH level, TBARS level, GR activity, CAT activity, SOD activity in liver, ↑ hepatic GPx activity, ↓ hepatic steatosis and ballooning, ↓ lobular and portal inflammation in liver | Noori et al., 2017 [48] | |
| Sprague-Dawley rats | Pomegranate juice (1 mL) or pomegranate seed extract (100 mg/mL) via oral administration, by force-feeding for 21 days | Streptozotocin-nicotinamide induced type 2 diabetes | ↔ b.w. gain, ↔ glycaemia and plasma insulin level, ↓ TC and TG (juice only), ↔ LDL-C and HDL-C (juice only), ↑ TC, LDL-C, and HDL-C (seed extract only), ↔ TG (seed extract only), ↓ plasma IL-6 and NF-κB levels, ↓ plasma TNF-α level (juice only), ↑ number and size of Islets of Langerhans (juice only) | Taheri Rouhi et al., 2017 [50] | |
| Neurodegenerative diseases | APPswe/PS1dE9 mice | Pomegranate extract (6.25 mL/L) via drinking water for 3 months | Transgenic model overexpressing APP, developing amyloid plaques and progressive cognitive deficits | ↑ behavioural performance, ↓ TNF-α in spleen and brain, ↓ NFATc1 activation in spleen and brain, ↑ p-NFATc2/NFATc2 ratio in brain, ↓ p-IκB/IκB ratio in brain, ↓ plaques in brain | Rojanathammanee et al., 2013 [59] |
| Lewis rats | Pomegranate juice (juice diluted 1:40 in water, equivalent to ~0.6–0.7 mg polyphenols) via drinking water for 2 weeks | Rotenone-induced degeneration of neurones | ↓ rearing behaviour, ↔ postural instability, ↔ catecholamine levels, ↓ dopamine fibres in striatum, ↓ nigral dopaminergic neurones, ↑ nitrotyrosine in substantia nigra, ↑ iNOS induction, ↑ NF-κB activation, ↑ caspase activation, ↔ IL-1β, TNF-α, and COX-2 protein expression | Tapias et al., 2014 [60] | |
| C57BL/6 mice | Pomegranate seed oil as emulsified nanodroplets (10 μL) via gavage for 10 days | MOG-induced experimental autoimmune encephalomyelitis | ↓ demyelination and oxidation of brain lipids, ↓ MDA in brain | Binyamin et al., 2015 [54] | |
| APPsw/Tg2576 mice | Pomegranate fruit (4% w/w) via chow for 15 months | Transgenic model overexpressing APP, developing amyloid plaques and progressive cognitive deficits | ↓ IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, and eotaxin levels in serum, ↓ Aβ-1 40 and 42 levels in brain, ↑ ATP levels the cortex and hippocampus, ↓ IL-1β, IL-6, TNF-α levels in cortex and hippocampus | Essa et al., 2015 [56] | |
| APPsw/Tg2576 mice | Pomegranate fruit (4% w/w) via chow for 15 months | Transgenic model overexpressing APP, developing amyloid plaques and progressive cognitive deficits | ↓ expression of genes IL-1β, IL-10, TNF-α, IGF-1, iNOS, and CCL2, ↑ BDNF gene expression, ↑ PSD-95, Munc18-1, SNAP25, and synaptophysin protein expression, ↑ p-CaMKIIα/CaMKIIα protein expression, ↑ p-CREB/CREB protein expression, ↑ BECN1 protein expression, ↑ LC3-I and LC3-II protein expression, ↑ Akt and mTOR protein expression, ↑ p70-S6K protein expression, ↔ APP and CTF-α protein expression, ↓ BACE-1, CTF-β, and sAPP-β protein expression, ↔ ADAM-10 and ADAM-17 protein expression | Braidy et al., 2016 [55] | |
| Wistar rats and mice | EA (10, 30, and 100 mg/kg b.w.) via intraperitoneal injection in a single administration | Scopolamine- and diazepam-induced cognitive impairments | ↓ amnesia in EPM and PA tests in mice ([EA] ≥ 30 mg/kg), ↓ amnesia in EPM test in rats ([EA] ≥ 30 mg/kg) | Mansouri et al., 2016 [57] | |
| C57Bl/6 mice | Pomegranate peel extract as microparticles (800 mg/kg b.w.) via oral administration for 35 days | Amyloid-β peptide-induced neurodegeneration | ↔ locomotor activity in an activity cage, ↔/↑ spatial memory in the Barnes maze, ↓ senile plaques, ↑ BDNF level in cortex and hippocampus, ↓ acetylcholinesterase activity in cortex and hippocampus, ↓ MDA in liver, ↔ SOD activity in hippocampus, cortex and serum, ↓ TNF-α in cortex, ↔ TNF-α in serum | Morzelle et al., 2016 [58] | |
| ICR mice | PUNI (1.5 mg/kg b.w.) via drinking water for 4 weeks | LPS-induced cognitive impairment | ↓ Aβ and BACE-1 protein expression, ↓ GFAP and AIF-1 protein expression, ↓ IL-1β, IL-6, and TNF-α release, ↑ GSH/GSSG ratio, ↓ ROS level, ↓ MDA, ↓ IκB phosphorylation, ↓ p50 and p65 protein expression | Kim et al., 2017 [53] | |
| RA | DBA/1 Lac J mice | POMxTM extract (13.6 or 34 mg/kg b.w.) via oral gavage for 10 days | Collagen-induced arthritis with chicken CII (Chondrex) | ↓ incidence and delay of arthritis, ↓ synovitis, ↓ pannus formation, ↓ joint degradation, ↓ IL-1β expression in ankle joints (13.6 mg/kg only), IL-6 expression in ankle joints, ↓ TNF-α expression in ankle joints (34 mg/kg only) | Shukla et al., 2008 [65] |
| Hepatocellular carcinoma | Sprague-Dawley rats | Pomegranate emulsion (1 or 10 g/kg b.w.) via oral gavage for 4 weeks prior to the DENA exposure and 18 weeks later | DENA-induced hepatocarcinogenesis | ↓ cyclin D1 expression (10 g/kg only), ↑ Bax/Bcl-2 ratio (10 g/kg only), ↓ β-catenin expression (10 g/kg only), ↑ GSK-3 expression (10 g/kg only) | Bhatia et al., 2013 [67] |
| Prostatic hyperplasia | Sprague-Dawley rats | Pomegranate fruit extract (25, 50, and 100 mg/kg b.w.) via oral gavage for 4 weeks | Testosterone-induced prostatic hyperplasia | ↓ prostate weight, ↓ PAP activity, ↑ GSH, ↔ total glutathione, ↑ SOD activity (100 mg/kg only), ↔ CAT activity, ↓ MDA, ↓ iNOS and COX-2 expression, ↔ AR, NF-κB, ER-α, and p-Akt expression | Ammar et al., 2015 [68] |
| Prostate cancer | Athymic nude mice | Pomegranate fruit extract (0.1% and 0.2% w/v) via oral administration for 28–51 days (until the implanted tumour reached to a volume of 1200 mm3) | Implantation with androgen-responsive CWR22Rn1 cells | ↓ PSA secretion | Malik & Mukhtar 2006 [69] |
↑: increase; ↔ : no change; ↓: decrease; ACAD: acyl coenzyme A dehydrogenase; ACC1: acetyl-CoA carboxylase 1; ACLY: ATP citrate lyase; ADAM: ADAM metallopeptidase; AIF-1: allograft inflammatory factor 1; Akt: protein kinase B; ALP: alkaline phosphatase; ALT: alanine transaminase; apoA: apolipoprotein A; apoB: apolipoprotein B; apoE: apolipoprotein E; APP: amyloid precursor protein; AR: androgen receptor; AST: aspartate aminotransferase; ATP: adenosine triphosphate; Aβ: amyloid β-peptides; b.w.: body weight; BACE-1: β-secretase 1; BAL: bronchoalveolar lavage; Bax: bcl-2-like protein 4; Bcl-2: B-cell lymphoma 2; Bdkrb: bradykinin receptor; BDNF: brain-derived neurotrophic factor; BECN1: beclin-1; BPM: beats per minute; CaMKIIα: calcium/calmodulin-dependent protein kinase type II α chain; CAT: catalase; CCL2: chemokine (C-C motif) ligand 2; CD36: cluster of differentiation 36; CMDI: colon mucosal damage index; COX-2: cyclooxygenase-2; CPT1: carnitine palmitoyl-transferase 1; CREB: cAMP response element-binding protein; CRP: C-reactive protein; CTF: C-terminal fragment of APP; CWR22Rn1: human prostate cancer cell line; DAI: disease activity index; DBP: diastolic blood pressure; DENA: diethyl-nitrosamine; DGAT: diglyceride acyltransferase; DNBS: 2,4-dinitro benzene sulfonic acid; DSS: dextran sulphate sodium; EA: ellagic acid; ECG: electrocardiogram; eNOS: endothelial nitric oxide synthase; EPM: elevated plus maze; ERK: extracellular signal-regulated kinase; ER-α: oestrogen receptor α; FA: fatty acids; FABP4: fatty acid binding protein 4; FAS: fatty acid synthase; FFA: free fatty acids; Fn1: fibronectin; FRAP: ferric reducing ability of plasma; GAE: gallic acid equivalents; GFAP: glial fibrillary acidic protein; GGT: γ-glutamyl-transferase; GPAM: glycerol-3-phosphate acyltransferase; GPx: glutathione peroxidase; GR: glutathione reductase; GSH: reduced glutathione; GSSG: oxidised glutathione; h: hour(s); HDL-C: high-density lipoprotein-cholesterol; HO-1: heme oxygenase 1; HOMA-IR: homeostatic model assessment–insulin resistance; hs-CRP: high-sensitivity C-reactive protein; ICR: imprinting control region; IgA: immunoglobulin A; IGF-1: insulin-like growth factor 1; IgG: immunoglobulin G; IgM: immunoglobulin M; IL-1β: interleukin 1β; IL-2: interleukin 2; IL-3: interleukin 3; IL-4: interleukin 4; IL-5: interleukin 5; IL-6: interleukin 6; IL-9: interleukin 9; IL-10: interleukin 10; IL-18: interleukin 18; iNOS: inducible nitric oxide synthase; IκB: inhibitor of NF-κB; JNK: c-Jun N-terminal kinase; LC3: microtubule-associated protein 1α/1β-light chain 3; LC3-I: cytosolic form of LC3; LC3-II: LC3-phosphatidylethanolamine conjugated; LDH: lactate dehydrogenase; LDL-C: low-density lipoprotein-cholesterol; LPS: lipopolysaccharide; m: month(s); MAP: mean arterial pressure; MCP-1: monocyte chemoattractant protein-1; MDA: malondialdehyde; MOG: myelin oligodendrocyte glycoprotein; MPO: myeloperoxidase; mTOR: mechanistic target of rapamycin; Munc-18: syntaxin binding protein 1; NAFLD: non-alcoholic fatty liver disease; NEFA: non-esterified fatty acids; NFATc: nuclear factor of activated T-cells, cytoplasmic; NF-κB: nuclear factor κ light-chain-enhancer of activated B cells; NO: nitric oxide; NQO-1: NAD(P)H quinone dehydrogenase 1; Nrf2: nuclear factor (erythroid-derived-2)-like 2; ObR: leptin receptor; p-: phosphorylated; p50: p50 protein; p65: p 65 protein; p70-S6K: ribosomal protein S6 kinase β-1; PA: passive avoidance; PAP: prostatic acid phosphatase; PBS: phosphate-buffered saline; PGC-1-: peroxisomal proliferator-activated receptor-γ coactivator-1; PGE2: prostaglandin E2; PON-1: paraoxonase 1; PPAR-α: peroxisome proliferator-activated receptor α; PPAR-γ: peroxisome proliferator-activated receptor γ; PSA: prostate-specific antigen; PSD-95: postsynaptic density protein 95; PTGES: prostaglandin E synthase; PUNI: punicalagin; ROS: reactive oxygen species; rpS6: ribosomal protein S6; SAA: serum amyloid A; sAPP-β: soluble APP-β; SBP: systolic blood pressure; SCD1: stearoyl-coenzyme A desaturase 1; SNAP25: synaptosomal-associated protein 25; SOD: superoxide dismutase; SR-BI: scavenger receptor class B type I; SREBP-1c: sterol regulatory element-binding protein 1c; STAT3: signal transducer and activator of transcription 3; TC: total cholesterol; TG: triglycerides; TGF-β1: transforming growth factor β1; TNBS: 2,4,6-trinitrobenzenesulfonic acid; TNF-α: tumour necrosis factor α; TSP-1: thrombospondin 1; UCP2: mitochondrial uncoupling protein 2; w/v: weight/volume; w/w: weight/weight.
These studies found anti-inflammatory effects of pomegranate and its biologically active compounds. The inflammation-related endpoints measured varied between local—e.g., macroscopic and histological examinations—to systemic evaluations—scoring systems, and plasma cytokines and CRP levels. Inflammation can also be induced by oxidative stress and, accordingly, oxidative markers were often evaluated. Among them, the most commonly measured endpoints included the formation of malondialdehyde (MDA) [22,26,27,53,54,58,68], antioxidant capacity (measured as FRAP or ferric reducing ability of plasma) [22], and the activities of glutathione peroxidase (GPx) [48], superoxide dismutase (SOD) [26,42,48,52,58,68], catalase (CAT) [48,68], and myeloperoxidase (MPO) [20,24,25,26,27]. Typically, the reduction of local inflammation and oxidative stress has also been reported to have more systemic effects.
Administration of pomegranate-derived products has been shown to reduce local inflammation in the bronchoalveolar tissue of COPD model mice [17,18,19] and in the joints of RA model mice [65]. There also exists a strong base of evidence suggesting that pomegranate extract exerts anti-inflammatory effects that may alleviate the symptoms of IBD [20,21,22,23,24,25,26,27], as colon tissue damage [20,22,23,24,25,26,27], antioxidant status [20,22,24,25,26,27], and inflammation [21,22,23,24,25,26] were all ameliorated by pomegranate fruit supplementation in rodent models of IBD. The mechanisms involved appear to be related to the inhibition of NF-κB [23,24,25,26], c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and signal transducer and activator of transcription 3 (STAT3) phosphorylation [23,24,25] in colon tissue.
Several groups have studied the effects of pomegranate on the prevention and amelioration of atherosclerosis and other CVD symptoms. de Nigris et al. (2007) [43] reported that supplementation with pomegranate juice or pomegranate fruit extract decreased the expression of vascular inflammation markers and transforming growth factor β-1 (TGFβ-1), and, likewise, increased endothelial NO synthase (eNOS) levels in a rat model of metabolic syndrome. Additionally, Labsi et al. (2016) [74] showed that intraperitoneal treatment with pomegranate peel extract for two months after the induction of echinococcosis significantly reduced the nitric oxide (NO) and TNF-α levels in Swiss albino mice. Pomegranate juice supplementation also ameliorated cardiac hypertrophy, and reduced oxidative stress and expression levels of interleukin 1β (IL-1β) and several fibrotic markers in the aorta of rats exposed to cigarette smoke [39]. Similarly, pomegranate juice supplementation slowed the development of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in a diet-induced obesity rat model [48]. Other authors illustrated the hypoglycaemic activity of pomegranate flowers [45], seeds [46], peel [45], and juice [41,48] in addition to its bioactive compound EA [49] using various rodent models of diabetes. Here, pomegranate-derived products appeared to regulate the activation of peroxisome proliferator-activated receptor γ (PPAR-γ) [46], a known regulator of fatty acid storage and glucose metabolism.
Several studies assessed the efficacy of pomegranate fruit as an antiproliferative agent in animal models of prostate hyperplasia and carcinoma. Malik and Mukhtar (2005) [69] demonstrated that, in vivo, oral administration of pomegranate fruit extract results in tumour growth inhibition accompanied by a reduction in serum prostate-specific antigen (PSA) levels. The decreasing of serum PSA levels have also been confirmed in two studies in humans [75,76]. In addition, dietary supplementation of 4% pomegranate extract with a standard chow diet inhibited neuro-inflammation in a transgenic mouse model of Alzheimer’s disease (AD). A delay in the formation of senile plaques and the loss of synaptic proteins was also observed [55,56]. Conversely, treatment with pomegranate juice did not protect neuronal degeneration in a separate study that used a rat model of AD, but instead exacerbated neuronal cell death and inflammation [60].
In conclusion, these studies using animal models of inflammation-related disease offer a crucial step in answering phenomenological questions related to the pathology of CID, but cannot be fully applied clinically due to the relatively high doses of pomegranate products used.
3.3. In Vitro Anti-Inflammatory Activity of Pomegranate Extracts or Pomegranate-Derived Bioactive Compounds
The anti-inflammatory effects observed in vivo have generally been confirmed in vitro. However, experimental conditions applied to cell cultures also tend to vary considerably with regards to the concentration of pomegranate extracts and time-points used (Table 5). In vitro, several pomegranate products have been tested including whole juice [61,63,70], extracts from the husk [29], seeds [62], or pulp [61,77], bioactive compounds present in pomegranate juice (ET [29,53,77] and EA [30]), or POMxTM extract [31]. Of all the groups working with these compounds, only Giménez-Bastida et al. (2012) [28] decided to assess the effects in intestinal cells of EA, which is present at high concentrations in the human colon. It is well known that pomegranate ETs are first hydrolysed to EA followed by transformation into the metabolite—urolithin—in the gut [5]. The importance of identifying bioactive metabolites using cell-based experimentation has been recently elaborated upon by Aragonès et al. (2017) [78].
Table 5.
Summary of the anti-inflammatory effects of pomegranate extracts or pomegranate-derived bioactive compounds assayed in cell culture studies.
| Cell Model | Primary Cell/Cell Line | Tested Compound(s), Dose, Duration | Pro-Inflammatory Treatment | Biological Effects | Reference |
|---|---|---|---|---|---|
| Intestinal cells | CCD18-Co | Uro-A (40 μM) + Uro-B (5 μM) + EA (1 μM) for 12–48 h in concomitant exposure with pro-inflammatory stimulus | IL-1β (1 ng/mL) or TNF-α (50 ng/mL) | ↓ IL-8 release, ↓ PGE2 release (only upon IL-1β stimulus), ↓ PAI-1 release, ↔ ICAM-1 and VCAM-1 release, ↔ MCP-1, ↓ cell migration and adhesion | Giménez-Bastida et al., 2012 [28] |
| Caco-2 | Pomegranate husk extract (containing 8.1 μM PUNI and 7.9 μM EA) or PUNI (50 μM) for 1 h as pre-treatment and 24 h in concomitant exposure with pro-inflammatory stimulus | basolateral side: IL-1β (25 μg/L) + TNF-α (50 μg/L) + IFN-γ (50 μg/L); apical side: LPS (1 mg/L) | ↓ IL-6 and MCP-1transcription, ↔ IL-8 transcription, ↓ IL-6, IL-8, and MCP-1 secretion | Hollebeeck et al., 2012 [29] | |
| Immune cells | KU812 | POMxTM extract (20, 40, and 100 μg/mL) for 2 h prior to pro-inflammatory stimulus | PMA (40 nM) + A23187 (1 μM) | ↓ IL-6 and IL-8 transcription, ↓ IL-6 and IL-8 secretion, ↓ JNK and ERK phosphorylation, ↓ NF-κB activation | Rasheed et al., 2009 [31] |
| Primary HGE | EA (12.5, 25, 50, and 100 μM) for 18 h | - | ↓ IL-8 transcription ([EA] ≥ 25 μM), ↑ BD2 transcription ([EA] ≥ 25 μM), ↑ SLPI transcription, ↓ CCL20 transcription ([EA] ≥ 50 μM), ↓ CXCL5 transcription ([EA] ≥ 50 μM), ↔ IL-1β secretion, ↑ IL-2 secretion ([EA] = 12.5 μM), ↓ IL-2 secretion ([EA] = 50 μM), ↔ IL-4, IL-6, and TNF-α secretion, ↓ IL-8 secretion ([EA] = 50 μM), ↔ MCP-1 secretion, ↑ CCL5 secretion ([EA] ≥ 12.5 μM), ↑ BD2 secretion ([EA] = 100 μM), ↔ SLPI secretion | Promsong et al., 2015 [30] | |
| Neuronal cells | PC12 | Pulp aqueous extract (6.25–800 μg/mL), pulp hydro-alcoholic extract (6.25, 12.5, 25, 50, 100, 200, 400, and 800 μg/mL), PJ extract (6.25, 12.5, 25, 50, 100, 200, 400, and 800 μg/mL) for 2 h prior glucose deprivation | Serum glucose deprivation | ↓ DNA damage ([PJ] ≥ 400 μg/mL) | Forouzanfar et al., 2013 [61] |
| BV-2 | Pomegranate seed oil (25 μg/mL) for 24 h | LPS (1 mg/mL) | ↓ NO production, ↓ TNF-α release, ↓ iNOS induction, ↓ caspase 3 activation | Račková et al., 2014 [62] | |
| SK-N-SH | PJ extract (25, 50, 100, and 200 μg/mL) for 24 h | IL-1β (10 U/mL) | ↓ PGE2 release, ↓ COX-2 protein expression, ↓ BACE-1 ([PJ] ≥ 50 μg/mL), ↓ amyloid-β ([PJ] ≥ 100 μg/mL), ↓ IκBα phosphorylation ([PJ] ≥ 50 μg/mL) | Velagapudi et al., 2016 [63] | |
| Primary astrocytes and BV-2 | PUNI (10, 20, and 50 μM) for 1 h | LPS (1 mg/mL) | ↓ iNOS and COX-2 protein expression, ↓ APP and BACE-1 protein expression, ↓ IκBα phosphorylation | Kim et al., 2017 [53] | |
| Rheumatoid arthritis cells | MH7A | Delphinidin (10 and 30 μM) for 24 h or 2 h (for ELISA) | TNF-α (20 ng/mL) | ↓ IL-1β and IL-6 expression, ↓ COX-2 expression, ↓ p65 acetylation, ↓ NF-κB DNA binding activity | Seong et al., 2011 [66] |
| Cancer cells | DU145 and PC3 | PJ (1% or 5%) for 18 h | - | ↓ IL-6 and IL-12 secretion, ↓ IL-1β secretion (DU145 only), ↓ CCL5 secretion (PC3 only) | Wang et al., 2011 [70] |
↑: increase; ↔ : no change; ↓: decrease; APP: amyloid precursor protein; BACE-1: β-secretase 1; BD2: β-defensin-2; BV-2: murine microglial cell line; Caco-2: human colorectal adenocarcinoma cell line; CCD18-Co: human colon cell line; CCL5: chemokine (C-C motif) ligand 5; CCL20 chemokine (C-C motif) ligand 20; COX-2: cyclooxygenase-2; CXCL5: chemokine (C-X-C motif) ligand 5; DU145: human prostate carcinoma cell line; EA: ellagic acid; ELISA: enzyme-linked immunosorbent assay; ERK: extracellular signal–regulated kinase; h: hour(s); ICAM-1: intracellular adhesion molecule-1; IFN-γ: interferon γ; IL-1β: interleukin 1β; IL-2: interleukin 2; IL-4: interleukin 4; IL-6: interleukin 6; IL-8: interleukin 8; iNOS: inducible nitric oxide synthase; IκB: inhibitor of NF-κB; JNK: c-Jun N-terminal kinase; KU812: human basophilic leukaemia cell line; LPS: lipopolysaccharide; MCP-1: monocyte chemoattractant protein-1; MH7A: human rheumatoid arthritis synovial cell line; NF-κB: nuclear factor κ light-chain-enhancer of activated B cells; NO: nitric oxide; P65: transcription factor p65; PAI-1: plasminogen activator inhibitor-1; PC3: human prostate cancer cell line; PC12: rat adrenal gland cell line; PGE2: prostaglandin E2; PJ: pomegranate juice; PMA: phorbol 12-myristate 13-acetate; PUNI: punicalagin; SK-N-SH: human neuroblastoma cell line; SLPI: secretory leukocyte protease inhibitor; TNF-α: tumour necrosis factor α; Uro-A: urolithin A; Uro-B: urolithin B; VCAM-1: vascular cell adhesion molecule-1.
Throughout our literature review, we found that the concentrations of the compounds applied to cells (mainly ET and EA) ranged from 1 to 100 μM; concentrations like 100 μM are considered high but physiologically attainable in the gut. The duration of supplementation was also variable, as groups used short-term (1–2 h) and long-term exposure to ET, EA, and pomegranate extracts (24–48 h), and were tested prior to exposure or in combination with pro-inflammatory stimuli, such as IL-1β [28,29,63], TNF-α [28,66], interferon γ (IFN-γ) [29], lipopolysaccharide (LPS) [29,53,62], phorbol 12-myristate 13-acetate (PMA) [31,79], or glucose deprivation [61]. In a few cases, pomegranate extracts or bioactive molecules were tested individually [30,70].
As mentioned above, in vivo studies have defined a clear role for NF-κB in the modulation of inflammation by pomegranate extracts, a finding that appears to be confirmed in vitro. Pomegranate juice [63], POMxTM extract [31], and their bioactive compounds—punicalagin [53] or delphinidin [66]—all suppressed NF-κB activation in various types of cells. It was found that ET reduced the expression of NF-κB target genes, including IL-6 and interleukin 8 (IL-8), upon exposure to pro-inflammatory stimuli in intestinal cells [29], while EA [30] and POMxTM [31] reduced NF-κB activation in various subsets of immune cells, and anthocyanin delphinidin reduced inflammation in rheumatoid arthritis cells [66]. Taken together, these results suggest that ET and other bioactive compounds present in pomegranate juice show anti-inflammatory effects in vitro, and that the mechanisms involved appear to be related to inactivation of NF-κB signalling.
4. Conclusions and Future Directions
Despite abundant literature on the putative effects of pomegranate fruit or extract on CID and other inflammation-related diseases, a definitive relationship between the consumption of pomegranate products and its beneficial properties has not yet been established. It is likely that the effects are due to the ingestion of pomegranate’s bioactive polyphenolic molecules. To date, most scientific research on the promising health benefits of pomegranate have been carried out in animal or cell culture models. Clinical trials are currently being conducted to examine a wide range of the potential health effects of pomegranate. However, these trials are few. The most promising properties of pomegranate thus far are related to its effects on diabetes, metabolic syndrome, and cardiovascular diseases. Further studies are required to determine the specific effects of ET-containing foods [80], and to explain the health benefits of pomegranate on CID.
Acknowledgments
This study was supported by the Italian Ministry of Education, University and Research MIUR – SIR Programme (No. RBSI14LHMB, funded to F.D.) and the Auckland Division, Cancer Society of New Zealand (funding to L.R.F.).
Author Contributions
F.D. and L.R.F. performed the research, analysed the data, and F.D. wrote the first draft of the paper. Both authors edited and contributed to drafts of the manuscript, and approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Nasef N.A., Mehta S., Ferguson L.R. Susceptibility to chronic inflammation: An update. Arch. Toxicol. 2017;91:1131–1141. doi: 10.1007/s00204-016-1914-5. [DOI] [PubMed] [Google Scholar]
- 2.Calder P.C. Inflammation: An introduction. In: Garg M., Wood L.G., editors. Nutrition and Physical Activity in Inflammatory Diseases. CABI; Wallingford, UK: 2013. pp. 1–22. [Google Scholar]
- 3.Calder P.C., Albers R., Antoine J.M., Blum S., Bourdet-Sicard R., Ferns G.A., Folkerts G., Friedmann P.S., Frost G.S., Guarner F., et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009;101:S1–S45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- 4.Landete J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011;44:1150–1160. doi: 10.1016/j.foodres.2011.04.027. [DOI] [Google Scholar]
- 5.Del Rio D., Rodriguez-Mateos A., Spencer J.P., Tognolini M., Borges G., Crozier A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sahebkar A., Ferri C., Giorgini P., Bo S., Nachtigal P., Grassi D. Effects of pomegranate juice on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2017;115:149–161. doi: 10.1016/j.phrs.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Lynn A., Hamadeh H., Leung W.C., Russell J.M., Barker M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012;67:309–314. doi: 10.1007/s11130-012-0295-z. [DOI] [PubMed] [Google Scholar]
- 8.Esmaillzadeh A., Tahbaz F., Gaieni I., Alavi-Majd H., Azadbakht L. Cholesterol-lowering effect of concentrated pomegranate juice consumption in type II diabetic patients with hyperlipidemia. Int. J. Vitam. Nutr. Res. 2006;76:147–151. doi: 10.1024/0300-9831.76.3.147. [DOI] [PubMed] [Google Scholar]
- 9.Rock W., Rosenblat M., Miller-Lotan R., Levy A.P., Elias M., Aviram M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008;56:8704–8713. doi: 10.1021/jf801756x. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Ortiz M., Martinez-Abundis E., Espinel-Bermudez M.C., Perez-Rubio K.G. Effect of pomegranate juice on insulin secretion and sensitivity in patients with obesity. Ann. Nutr. Metab. 2011;58:220–223. doi: 10.1159/000330116. [DOI] [PubMed] [Google Scholar]
- 11.Al-Muammar M.N., Khan F. Obesity: The preventive role of the pomegranate (Punica granatum) Nutrition. 2012;28:595–604. doi: 10.1016/j.nut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Asgary S., Sahebkar A., Afshani M.R., Keshvari M., Haghjooyjavanmard S., Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014;28:193–199. doi: 10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- 13.Sumner M.D., Elliott-Eller M., Weidner G., Daubenmier J.J., Chew M.H., Marlin R., Raisin C.J., Ornish D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005;96:810–814. doi: 10.1016/j.amjcard.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Sahebkar A., Simental-Mendía L.E., Giorgini P., Ferri C., Grassi D. Lipid profile changes after pomegranate consumption: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2016;23:1103–1112. doi: 10.1016/j.phymed.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Sahebkar A., Gurban C., Serban A., Andrica F., Serban M.C. Effects of supplementation with pomegranate juice on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 2016;23:1095–1102. doi: 10.1016/j.phymed.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bachoual R., Talmoudi W., Boussetta T., Braut F., El-Benna J. An aqueous pomegranate peel extract inhibits neutrophil myeloperoxidase in vitro and attenuates lung inflammation in mice. Food Chem. Toxicol. 2011;49:1224–1228. doi: 10.1016/j.fct.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 18.De Oliveira J.F., Garreto D.V., da Silva M.C., Fortes T.S., de Oliveira R.B., Nascimento F.R., Da Costa F.B., Grisotto M.A., Nicolete R. Therapeutic potential of biodegradable microparticles containing Punica granatum L. (pomegranate) in murine model of asthma. Inflamm. Res. 2013;62:971–980. doi: 10.1007/s00011-013-0659-3. [DOI] [PubMed] [Google Scholar]
- 19.Husari A., Hashem Y., Bitar H., Dbaibo G., Zaatari G., El Sabban M. Antioxidant activity of pomegranate juice reduces emphysematous changes and injury secondary to cigarette smoke in an animal model and human alveolar cells. Int. J. Chron. Obstruct. Pulmon. Dis. 2016;11:227–237. doi: 10.2147/COPD.S97027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boussetta T., Raad H., Letteron P., Gougerot-Pocidalo M.A., Marie J.C., Driss F., El-Benna J. Punicic acid a conjugated linolenic acid inhibits TNFa-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS ONE. 2009;4:e6458. doi: 10.1371/journal.pone.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H., Banerjee N., Ivanov I., Pfent C.M., Prudhomme K.R., Bisson W.H., Dashwood R.H., Talcott S.T., Mertens-Talcott S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol. Nutr. Food Res. 2016;60:1912–1923. doi: 10.1002/mnfr.201501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larrosa M., González-Sarrías A., Yáñez-Gascón M.J., Selma M.V., Azorín-Ortuño M., Toti S., Tomás-Barberán F., Dolara P., Espín J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Marín M., Maria Giner R., Ríos J.L., Recio M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013;150:925–934. doi: 10.1016/j.jep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Rosillo M.A., Sánchez-Hidalgo M., Cárdeno A., Aparicio-Soto M., Sánchez-Fidalgo S., Villegas I., de la Lastra C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012;66:235–242. doi: 10.1016/j.phrs.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Rosillo M.A., Sánchez-Hidalgo M., Cárdeno A., de la Lastra C.A. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011;82:737–745. doi: 10.1016/j.bcp.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 26.Shah T.A., Parikh M., Patel K.V., Patel K.G., Joshi C.G., Gandhi T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol. Cell. Biochem. 2016;419:65–74. doi: 10.1007/s11010-016-2750-x. [DOI] [PubMed] [Google Scholar]
- 27.Singh K., Jaggi A.S., Singh N. Exploring the ameliorative potential of Punica granatum in dextran sulfate sodium induced ulcerative colitis in mice. Phytother. Res. 2009;23:1565–1574. doi: 10.1002/ptr.2822. [DOI] [PubMed] [Google Scholar]
- 28.Giménez-Bastida J.A., Larrosa M., González-Sarrías A., Tomás-Barberán F., Espín J.C., García-Conesa M.T. Intestinal ellagitannin metabolites ameliorate cytokine-induced inflammation and associated molecular markers in human colon fibroblasts. J. Agric. Food Chem. 2012;60:8866–8876. doi: 10.1021/jf300290f. [DOI] [PubMed] [Google Scholar]
- 29.Hollebeeck S., Winand J., Hérent M.F., During A., Leclercq J., Larondelle Y., Schneider Y.J. Anti-inflammatory effects of pomegranate (Punica granatum L.) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012;3:875–885. doi: 10.1039/c2fo10258g. [DOI] [PubMed] [Google Scholar]
- 30.Promsong A., Chung W.O., Satthakarn S., Nittayananta W. Ellagic acid modulates the expression of oral innate immune mediators: Potential role in mucosal protection. J. Oral Pathol. Med. 2015;44:214–221. doi: 10.1111/jop.12223. [DOI] [PubMed] [Google Scholar]
- 31.Rasheed Z., Akhtar N., Anbazhagan A.N., Ramamurthy S., Shukla M., Haqqi T.M. Polyphenol-rich pomegranate fruit extract (POMx) suppresses PMACI-induced expression of pro-inflammatory cytokines by inhibiting the activation of MAP Kinases and NF-κB in human KU812 cells. J. Inflamm. 2009;6 doi: 10.1186/1476-9255-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asghari G., Sheikholeslami S., Mirmiran P., Chary A., Hedayati M., Shafiee A., Azizi F. Effect of pomegranate seed oil on serum TNF-a level in dyslipidemic patients. Int. J. Food Sci. Nutr. 2012;63:368–371. doi: 10.3109/09637486.2011.631521. [DOI] [PubMed] [Google Scholar]
- 33.Asgary S., Keshvari M., Sahebkar A., Hashemi M., Rafieian-Kopaei M. Clinical investigation of the acute effects of pomegranate juice on blood pressure and endothelial function in hypertensive individuals. ARYA Atheroscler. 2013;9:326–331. [PMC free article] [PubMed] [Google Scholar]
- 34.Shishehbor F., Mohammad Shahi M., Zarei M., Saki A., Zakerkish M., Shirani F., Zare M. Effects of concentrated pomegranate juice on subclinical inflammation and cardiometabolic risk factors for type 2 diabetes: A quasi-experimental study. Int. J. Endocrinol. Metab. 2016;14:e33835. doi: 10.5812/ijem.33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moazzen H., Alizadeh M. Effects of pomegranate juice on cardiovascular risk factors in patients with metabolic syndrome: A double-blinded, randomized crossover controlled trial. Plant Foods Hum. Nutr. 2017;72:126–133. doi: 10.1007/s11130-017-0605-6. [DOI] [PubMed] [Google Scholar]
- 36.Shema-Didi L., Sela S., Ore L., Shapiro G., Geron R., Moshe G., Kristal B. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: A randomized placebo-controlled trial. Free Radic. Biol. Med. 2012;53:297–304. doi: 10.1016/j.freeradbiomed.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Rivara M.B., Mehrotra R., Linke L., Ruzinski J., Ikizler T.A., Himmelfarb J. A pilot randomized crossover trial assessing the safety and short-term effects of pomegranate supplementation in hemodialysis patients. J. Ren. Nutr. 2015;25:40–49. doi: 10.1053/j.jrn.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu P.T., Fitschen P.J., Kistler B.M., Jeong J.H., Chung H.R., Aviram M., Phillips S.A., Fernhall B., Wilund K.R. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J. Med. Food. 2015;18:941–949. doi: 10.1089/jmf.2014.0103. [DOI] [PubMed] [Google Scholar]
- 39.Al Hariri M., Zibara K., Farhat W., Hashem Y., Soudani N., Al Ibrahim F., Hamade E., Zeidan A., Husari A., Kobeissy F. Cigarette smoking-induced cardiac hypertrophy, vascular inflammation and injury are attenuated by antioxidant supplementation in an animal model. Front. Pharmacol. 2016;7 doi: 10.3389/fphar.2016.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Jarallah A., Igdoura F., Zhang Y., Tenedero C.B., White E.J., MacDonald M.E., Igdoura S.A., Trigatti B.L. The effect of pomegranate extract on coronary artery atherosclerosis in SR-BI/APOE double knockout mice. Atherosclerosis. 2013;228:80–89. doi: 10.1016/j.atherosclerosis.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Betanzos-Cabrera G., Guerrero-Solano J.A., Martínez-Pérez M.M., Calderón-Ramos Z.G., Belefant-Miller H., Cancino-Diaz J.C. Pomegranate juice increases levels of paraoxonase1 (PON1) expression and enzymatic activity in streptozotocin-induced diabetic mice fed with a high-fat diet. Food Res. Int. 2011;44:1381–1385. doi: 10.1016/j.foodres.2011.01.042. [DOI] [Google Scholar]
- 42.Çukurova Z., Hergünsel O., Eren G., Gedikbaşi A., Uhri M., Demir G., Tekdöş Y. The effect of pomegranate juice on diabetes-related oxidative stress in rat lung. Türkiye Klinikeri J. Med. Sci. 2012;32:444–452. doi: 10.5336/medsci.2011-24472. [DOI] [Google Scholar]
- 43.de Nigris F., Balestrieri M.L., Williams-Ignarro S., D’Armiento F.P., Fiorito C., Ignarro L.J., Napoli C. The influence of pomegranate fruit extract in comparison to regular pomegranate juice and seed oil on nitric oxide and arterial function in obese Zucker rats. Nitric Oxide. 2007;17:50–54. doi: 10.1016/j.niox.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 44.Dushkin M., Khrapova M., Kovshik G., Chasovskikh M., Menshchikova E., Trufakin V., Shurlygina A., Vereschagin E. Effects of Rhaponticum carthamoides versus Glycyrrhiza glabra and Punica granatum extracts on metabolic syndrome signs in rats. BMC Complement. Altern. Med. 2014;14 doi: 10.1186/1472-6882-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harzallah A., Hammami M., Kępczyńska M.A., Hislop D.C., Arch J.R., Cawthorne M.A., Zaibi M.S. Comparison of potential preventive effects of pomegranate flower, peel and seed oil on insulin resistance and inflammation in high-fat and high-sucrose diet-induced obesity mice model. Arch. Physiol. Biochem. 2016;122:75–87. doi: 10.3109/13813455.2016.1148053. [DOI] [PubMed] [Google Scholar]
- 46.Hontecillas R., O’Shea M., Einerhand A., Diguardo M., Bassaganya-Riera J. Activation of PPAR g and a by punicic acid ameliorates glucose tolerance and suppresses obesity-related inflammation. J. Am. Coll. Nutr. 2009;28:184–195. doi: 10.1080/07315724.2009.10719770. [DOI] [PubMed] [Google Scholar]
- 47.Neyrinck A.M., Van Hée V.F., Bindels L.B., De Backer F., Cani P.D., Delzenne N.M. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: Potential implication of the gut microbiota. Br. J. Nutr. 2013;109:802–809. doi: 10.1017/S0007114512002206. [DOI] [PubMed] [Google Scholar]
- 48.Noori M., Jafari B., Hekmatdoost A. Pomegranate juice prevents development of non-alcoholic fatty liver disease in rats by attenuating oxidative stress and inflammation. J. Sci. Food Agric. 2017;97:2327–2332. doi: 10.1002/jsfa.8042. [DOI] [PubMed] [Google Scholar]
- 49.Panchal S.K., Ward L., Brown L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013;52:559–568. doi: 10.1007/s00394-012-0358-9. [DOI] [PubMed] [Google Scholar]
- 50.Taheri Rouhi S.Z., Sarker M.M., Rahmat A., Alkahtani S.A., Othman F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague-Dawley rats. BMC Complement. Altern. Med. 2017;17 doi: 10.1186/s12906-017-1667-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilahur G., Padró T., Casani L., Mendieta G., López J.A., Streitenberger S., Badimon L. Polyphenol-enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Rev. Esp. Cardiol. 2015;68:216–225. doi: 10.1016/j.recesp.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 52.Zou X., Yan C., Shi Y., Cao K., Xu J., Wang X., Chen C., Luo C., Li Y., Gao J., et al. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: The protective effects of pomegranate with its active component punicalagin. Antioxid. Redox Signal. 2014;21:1557–1570. doi: 10.1089/ars.2013.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim Y.E., Hwang C.J., Lee H.P., Kim C.S., Son D.J., Ham Y.W., Hellström M., Han S.B., Kim H.S., Park E.K., et al. Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-κB. Neuropharmacology. 2017;117:21–32. doi: 10.1016/j.neuropharm.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 54.Binyamin O., Larush L., Frid K., Keller G., Friedman-Levi Y., Ovadia H., Abramsky O., Magdassi S., Gabizon R. Treatment of a multiple sclerosis animal model by a novel nanodrop formulation of a natural antioxidant. Int. J. Nanomed. 2015;10:7165–7174. doi: 10.2147/IJN.S92704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braidy N., Essa M.M., Poljak A., Selvaraju S., Al-Adawi S., Manivasagm T., Thenmozhi A.J., Ooi L., Sachdev P., Guillemin G.J. Consumption of pomegranates improves synaptic function in a transgenic mice model of Alzheimer’s disease. Oncotarget. 2016;7:64589–64604. doi: 10.18632/oncotarget.10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Essa M.M., Subash S., Akbar M., Al-Adawi S., Guillemin G.J. Long-term dietary supplementation of pomegranates, figs and dates alleviate neuroinflammation in a transgenic mouse model of Alzheimer’s disease. PLoS ONE. 2015;10:e0120964. doi: 10.1371/journal.pone.0120964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mansouri M.T., Farbood Y., Naghizadeh B., Shabani S., Mirshekar M.A., Sarkaki A. Beneficial effects of ellagic acid against animal models of scopolamine- and diazepam-induced cognitive impairments. Pharm. Biol. 2016;54:1947–1953. doi: 10.3109/13880209.2015.1137601. [DOI] [PubMed] [Google Scholar]
- 58.Morzelle M.C., Salgado J.M., Telles M., Mourelle D., Bachiega P., Buck H.S., Viel T.A. Neuroprotective effects of pomegranate peel extract after chronic infusion with amyloid-b peptide in mice. PLoS ONE. 2016;11:e0166123. doi: 10.1371/journal.pone.0166123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rojanathammanee L., Puig K.L., Combs C.K. Pomegranate polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in vitro and in a transgenic mouse model of Alzheimer disease. J. Nutr. 2013;143:597–605. doi: 10.3945/jn.112.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tapias V., Cannon J.R., Greenamyre J.T. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol. Aging. 2014;35:1162–1176. doi: 10.1016/j.neurobiolaging.2013.10.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Forouzanfar F., Afkhami Goli A., Asadpour E., Ghorbani A., Sadeghnia H.R. Protective effect of Punica granatum L. against serum/glucose deprivation-induced PC12 cells injury. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/716730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Račková L., Ergin V., Burcu Bali E., Kuniaková M., Karasu Ç. Pomegranate seed oil modulates functions and survival of BV-2 microglial cells in vitro. Int. J. Vitam. Nutr. Res. 2014;84:295–309. doi: 10.1024/0300-9831/a000216. [DOI] [PubMed] [Google Scholar]
- 63.Velagapudi R., Baco G., Khela S., Okorji U., Olajide O. Pomegranate inhibits neuroinflammation and amyloidogenesis in IL-1b-stimulated SK-N-SH cells. Eur. J. Nutr. 2016;55:1653–1660. doi: 10.1007/s00394-015-0984-0. [DOI] [PubMed] [Google Scholar]
- 64.Ghavipour M., Sotoudeh G., Tavakoli E., Mowla K., Hasanzadeh J., Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017;71:92–96. doi: 10.1038/ejcn.2016.151. [DOI] [PubMed] [Google Scholar]
- 65.Shukla M., Gupta K., Rasheed Z., Khan K.A., Haqqi T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition. 2008;24:733–743. doi: 10.1016/j.nut.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seong A.R., Yoo J.Y., Choi K., Lee M.H., Lee Y.H., Lee J., Jun W., Kim S., Yoon H.G. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem. Biophys. Res. Commun. 2011;410:581–586. doi: 10.1016/j.bbrc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 67.Bhatia D., Thoppil R.J., Mandal A., Samtani K.A., Darvesh A.S., Bishayee A. Pomegranate bioactive constituents suppress cell proliferation and induce apoptosis in an experimental model of hepatocellular carcinoma: Role of Wnt/b-catenin signaling pathway. Evid. Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/371813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ammar A.E., Esmat A., Hassona M.D., Tadros M.G., Abdel-Naim A.B., Guns E.S. The effect of pomegranate fruit extract on testosterone-induced BPH in rats. Prostate. 2015;75:679–692. doi: 10.1002/pros.22951. [DOI] [PubMed] [Google Scholar]
- 69.Malik A., Mukhtar H. Prostate cancer prevention through pomegranate fruit. Cell Cycle. 2006;5:371–373. doi: 10.4161/cc.5.4.2486. [DOI] [PubMed] [Google Scholar]
- 70.Wang L., Alcon A., Yuan H., Ho J., Li Q.J., Martins-Green M. Cellular and molecular mechanisms of pomegranate juice-induced anti-metastatic effect on prostate cancer cells. Integr. Biol. 2011;3:742–754. doi: 10.1039/c0ib00122h. [DOI] [PubMed] [Google Scholar]
- 71.Kojadinovic M.I., Arsic A.C., Debeljak-Martacic J.D., Konic-Ristic A.I., Kardum N.D., Popovic T.B., Glibetic M.D. Consumption of pomegranate juice decreases blood lipid peroxidation and levels of arachidonic acid in women with metabolic syndrome. J. Sci. Food Agric. 2017;97:1798–1804. doi: 10.1002/jsfa.7977. [DOI] [PubMed] [Google Scholar]
- 72.Clinical Tricals.gov. [(accessed on 20 February 2017)]; Available online: http://www.clinicaltrials.gov.
- 73.Kaulmann A., Bohn T. Bioactivity of polyphenols: Preventive and adjuvant strategies toward reducing inflammatory bowel diseases-promises, perspectives, and pitfalls. Oxid. Med. Cell. Longev. 2016;2016:9346470. doi: 10.1155/2016/9346470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Labsi M., Khelifi L., Mezioug D., Soufli I., Touil-Boukoffa C. Antihydatic and immunomodulatory effects of Punica granatum peel aqueous extract in a murine model of echinococcosis. Asian Pac. J. Trop. Med. 2016;9:211–220. doi: 10.1016/j.apjtm.2016.01.038. [DOI] [PubMed] [Google Scholar]
- 75.Pantuck A.J., Leppert J.T., Zomorodian N., Aronson W., Hong J., Barnard R.J., Seeram N., Liker H., Wang H., Elashoff R., et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006;12:4018–4026. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 76.Paller C.J., Ye X., Wozniak P.J., Gillespie B.K., Sieber P.R., Greengold R.H., Stockton B.R., Hertzman B.L., Efros M.D., Roper R.P., et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:50–55. doi: 10.1038/pcan.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danesi F., Kroon P.A., Saha S., de Biase D., D’Antuono L.F., Bordoni A. Mixed pro- and anti-oxidative effects of pomegranate polyphenols in cultured cells. Int. J. Mol. Sci. 2014;15:19458–19471. doi: 10.3390/ijms151119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aragonès G., Danesi F., Del Rio D., Mena P. The importance of studying cell metabolism when testing the bioactivity of phenolic compounds. Trends Food Sci. Technol. 2017 doi: 10.1016/j.tifs.2017.02.001. [DOI] [Google Scholar]
- 79.Danesi F., Philpott M., Huebner C., Bordoni A., Ferguson L.R. Food-derived bioactives as potential regulators of the IL-12/IL-23 pathway implicated in inflammatory bowel diseases. Mutat. Res. 2010;690:139–144. doi: 10.1016/j.mrfmmm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 80.González-Sarrías A., García-Villalba R., Romo-Vaquero M., Alasalvar C., Örem A., Zafrilla P., Tomás-Barberán F.A., Selma M.V., Espín J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomised clinical trial. Mol. Nutr. Food Res. 2017;61 doi: 10.1002/mnfr.201600830. [DOI] [PubMed] [Google Scholar]