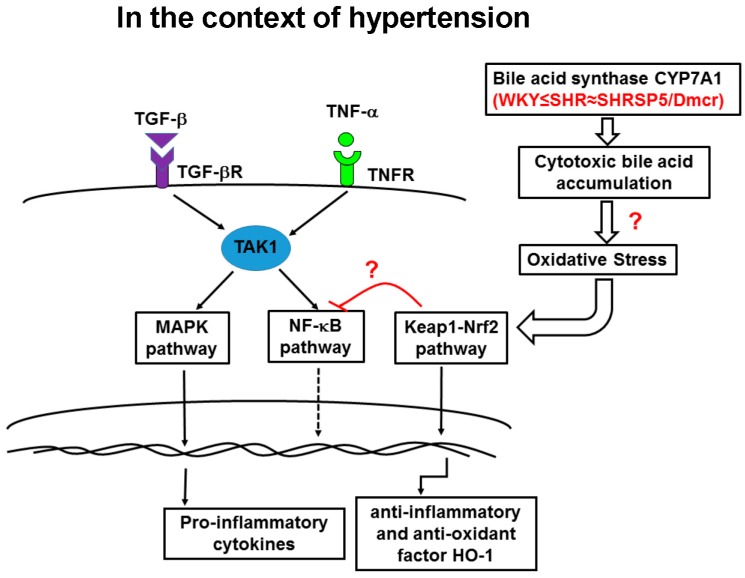
Figure 6.
Interactions among the MAPK, NF-κB, and Nrf2 signaling pathways contribute to the progression of HFC diet-induced NASH in the context of hypertension. The inflammatory cytokines, TGF-β1 and TNF-α, bind to their receptors and activate downstream signaling in the MAPK pathway, as well as NF-κB via TAK1, following HFC feeding, which may be responsible for the severe inflammatory response in the livers of the hypertensive strains. Compared with the normotensive rats, the HFC diet induced increased activation of the MAPK pathway in the hypertensive strains. The NF-κB pathway was activated in the initial stage of inflammation (after 2 weeks feeding with the HFC diet), but the activation appeared to be discontinued for unknown reasons in the following stage (8 and 14 weeks). The Nrf2 pathway was repressed following HFC feeding, which suppressed the upregulation of the anti-inflammatory and antioxidant factor HO-1, thereby suggesting that the antioxidant capacity was attenuated in the hypertensive strains. In addition, an overexpression of the bile acid synthase CYP7A1 in the hypertensive strains may lead to the accumulation of cytotoxic bile acids in the livers and cause oxidative stress, thereby inducing mild inflammatory response and activating the Nrf2/Keap1 pathway before feeding the HFC diet. Therefore, the combination of increased activation of the inflammatory signaling pathways and suppression of the antioxidant pathway after HFC feeding may have contributed to the exacerbation of hepatitis.