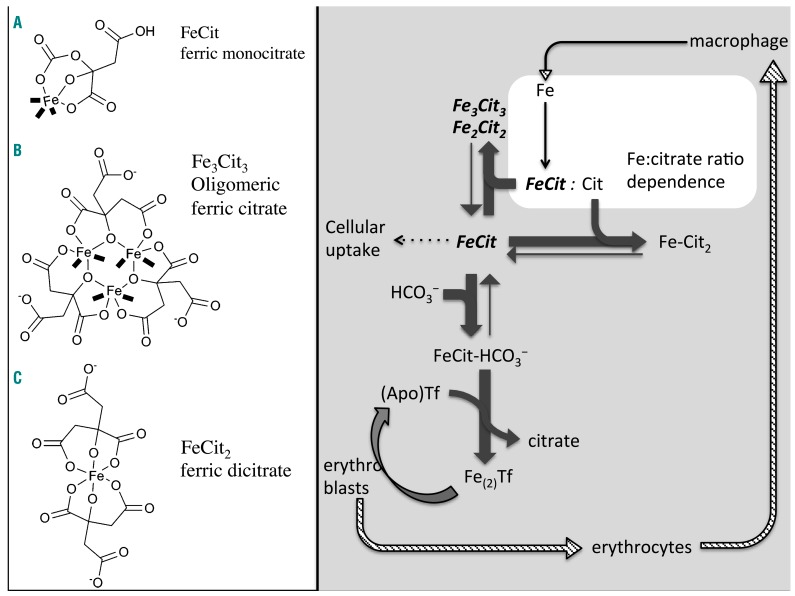
Figure 5.
Model of apotransferrin-dependent re-speciation of polymeric ferric citrate. The paradoxical effect of apotransferrin seen in Figure 4D, in which uptake from 10 μM ferric citrate is increased before it is abolished by apotransferrin, is consistent with the sub-equivalent concentration of apotransferrin disrupting ferric citrate oligomers and releasing from them ferric monocitrate species. As apotransferrin binds a portion of iron in high ratio ferric citrate, this decreases the amount of iron per citrate, so effectively changes the iron-to-citrate ratio, i.e. re-speciates it. In the presence of apotransferrin and bicarbonate, which forms a ternary complex with citrate, oligomer complexes of ferric citrate become a source of ferric monocitrate species. These are subject to competition with uptake mechanisms for cellular entry (dotted line), with apotransferrin for the formation of ferrotransferrin and with citrate for the formation of ferric dicitrate, citrate also competing with apotransferrin for ferric monocitrate. Kinetic differences between ferrotransferrin formation and cellular uptake from ferric citrate may explain the additional iron uptake from newly released mononuclear species before apotransferrin can chelate them altogether. The coordination sites on the iron (shown in bold) are typically occupied by water, but they are labile sites and can also bind oxygen and H2O2, rendering the species susceptible to redox chemistry (marked in bold cursive on the right). They are also the sites of condensation with other iron complexes.