Abstract
Asparaginase is an essential component of combination chemotherapy for childhood acute lymphoblastic leukemia and non-Hodgkin lymphoma. The value of asparaginase was further addressed in a group of non-very high-risk patients by comparing prolonged (long-asparaginase) versus standard (short-asparaginase) native E. coli asparaginase treatment in a randomized part of the phase III 58951 trial of the European Organization for Research and Treatment of Cancer Children’s Leukemia Group. The main endpoint was disease-free survival. Overall, 1,552 patients were randomly assigned to long-asparaginase (775 patients) or short-asparaginase (777 patients). Patients with grade ≥2 allergy to native E. coli asparaginase were switched to equivalent doses of Erwinia or pegylated E. coli asparaginase. The 8-year disease-free survival rate (±standard error) was 87.0±1.3% in the long-asparaginase group and 84.4±1.4% in the short-asparaginase group (hazard ratio: 0.87; P=0.33) and the 8-year overall survival rate was 92.6±1.0% and 91.3±1.2% respectively (hazard ratio: 0.89; P=0.53). An exploratory analysis suggested that the impact of long-asparaginase was beneficial in the National Cancer Institute standard-risk group with regards to disease-free survival (hazard ratio: 0.70; P=0.057), but far less so with regards to overall survival (hazard ratio: 0.89). The incidences of grade 3–4 infection during consolidation (25.2% versus 14.4%) and late intensification (22.6% versus 15.9%) and the incidence of grade 2–4 allergy were higher in the long-asparaginase arm (30% versus 21%). Prolonged native E. coli asparaginase therapy in consolidation and late intensification for our non-very high-risk patients did not improve overall outcome but led to an increase in infections and allergy. This trial was registered at www.clinicaltrials.gov as #NCT00003728.
Introduction
The rate of success in the treatment of children with acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoblastic lymphoma (NHL) has increased steadily since the 1970s. With contemporary risk-directed therapy the 5-year overall survival rate is nowadays nearly 90%.1 This major improvement in outcome results from a better understanding of the disease, more accurate stratification according to prognostic risk factors and optimized drug use.
One of the essential components in treatment protocols for childhood ALL and NHL is asparaginase (ASNase), an enzyme that catalyzes serum asparagine and glutamine deamination. In contrast to healthy cells, lymphoblasts are unable to produce endogenous asparagine because of the lack of the enzyme asparagine synthetase, and rely on plasma levels of this amino acid for protein synthesis. A depletion of plasma asparagine by ASNase results in selective apoptosis of lymphoblasts.2 The adverse effects of ASNase are mainly related to disturbances in normal protein synthesis and to hypersensitivity reactions.
Several study groups have demonstrated a benefit of intensive ASNase treatment compared to less intensive regimens.3–7 In the European Organization for Research and Treatment of Cancer Children’s Leukemia Group (EORTC-CLG) 58881 trial, our study group showed that the use of a more potent ASNase with a longer half-life improved patients’ outcome. Patients receiving native E. coli ASNase 10,000 IU/m2 twice weekly in induction and late intensification experienced longer event-free and overall survival than patients randomized to Erwinia ASNase at the same dosage and frequency.3,8 These results were confirmed by the DFCI 95-01 trial for patients treated with 25,000 IU/m2 weekly of native E. coli or Erwinia ASNase4. Both studies made clear that the use of native E. coli ASNase was responsible for a reduction in relapse rate, albeit resulting in more toxicity. Based on these studies and data from pharmacokinetic and ASNase activity monitoring, it is known that higher doses of Erwinia ASNase and shorter dose intervals are required to achieve ASNase activity that is adequate in comparison with that provided by E. coli ASNase. Several study groups now incorporate ASNase activity monitoring for the optimization of ASNase therapy.
Not only do the formulation and dose of ASNase seem to play a role, but the duration of the ASNase treatment also has an impact on survival. The Italian, Dutch and Hungarian IDH-ALL-91 trial demonstrated an improved outcome for patients who received extended high-dose native E. coli ASNase treatment in consolidation therapy (25,000 IU/m2 weekly for 20 weeks) compared to the same treatment based on a reduced-intensity Berlin-Frankfurt-Münster (BFM)-backbone without extra ASNase.5 The benefit of prolonged native E. coli ASNase for T-cell ALL and NHL was also proven in the Pediatric Oncology Group (POG) study 8704 in which a high-dose ASNase regimen of 25,000 IU/m2 given weekly for 20 weeks starting from consolidation resulted in higher continuous complete remission rates.6 An increase in event-free survival was also observed in the subsequent Dana Farber Cancer Institute (DFCI) trial (ALL 91-01) as a result of the prolongation of high-dose native E. coli ASNase (25,000 IU/m2 weekly) or pegylated (PEG)-ASNase (2,500 IU/m2 every other week) for 20 to 30 weeks during intensification therapy.7 However, patients were not randomized to a standard versus prolonged schedule of ASNase, and the duration of ASNase was actually based on ASNase tolerance.
In contrast, the controversy of intensified ASNase treatment was highlighted in the Associazione Italiana Ematologia e Oncologia Pediatrica (AIEOP) ALL-91 trial, in which high-dose E. coli ASNase treatment (25,000 IU/m2 weekly for 20 weeks during late intensification and early continuation) did not have an impact on disease-free survival compared to standard E. coli ASNase treatment (4 doses of 10,000 IU/m2 during late intensification).9
The mode of administration (continuous or discontinuous ASNase administration) may have different implications on the development of ASNase hypersensitivity and silent inactivation of ASNase. The probability of the appearance of hypersensitivity reactions does not only increase with the number of administrations within the same cycle but also in discontinuous administration schedules, in which ASNase is reintroduced after an ASNase-free interval.10
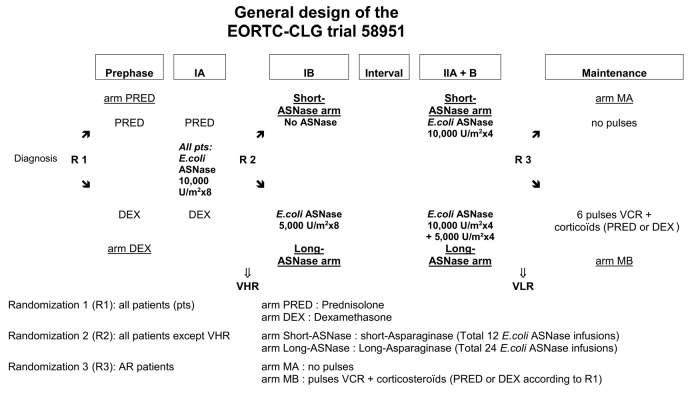
Furthermore, more intensive ASNase therapy goes along with an increase in ASNase-related toxicities. With the advantages and side effects of ASNase in mind, the optimal dosage and number of ASNase administrations remain subject of debate. The EORTC-CLG, therefore, conducted a randomized phase III trial 58951, comparing a conventional native E. coli ASNase regimen with a prolonged native E. coli ASNase therapy in a BFM-based treatment (Figure 1).
Figure 1.
General scheme of the EORTC-CLG 58951 trial. The EORTC-CLG trial 58951 embedded three main randomized comparisons: [R1] the value of prednisolone (PRED, 60 mg/m2/day) versus dexamethasone (DEX, 6 mg/m2/day) in induction for all patients;12 [R2] the value of prolonged courses of ASNase throughout consolidation and late intensification for all non-very high risk (non-VHR) patients; and [R3] the value of vincristine (VCR) and corticosteroid pulses introduced in continuation therapy for average risk (AR) patients.13 IA: induction phase; IB: consolidation phase; II A+B: late intensification phase; VCR: vincristine; VLR: very low risk (group); AR: average risk (group); VHR: very high risk (group).
Methods
Patients
As previously described,12,13 the EORTC-CLG 58951 protocol included all children aged less than 18 years, with previously untreated ALL of French-American-British (FAB) L1 or L2 morphology whatever the immuno-phenotype, or precursor B- or T-lymphoblastic NHL. Patients with ALL of FAB L3 morphology and diffuse large cell B-cell lymphoma, Burkitt lymphoma or high-grade B-cell lymphoma Burkitt-like, were excluded. Infants (<1 year) and patients with Philadelphia-positive ALL were allocated to separate disease-specific protocols (Interfant protocol for infants and Esphall protocol for Philadelphia-positive patients).
As described in the Online Supplementary Appendix, patients were assigned to different risk groups: very low risk, average risk low, average risk high and very high risk.12,13
Before entering the study, informed consent was obtained from the parents or legal guardians according to the Declaration of Helsinki. Both the EORTC Protocol Review Committee and the local institutional ethical committees of each participating center approved the study design.
Treatment and study design
The EORTC-CLG 58951 study was based on a BFM-like protocol with a prephase, four-drug induction (IA), consolidation phase (IB), interval phase with central nervous system-directed treatment (without cranial radiotherapy), late intensification (IIA+B) and maintenance therapy (Figure 1). Online Supplementary Table S1A,B outlines the study design of the EORTC-CLG 58951 study for the very low risk, average risk low and average risk high risk groups.12,13
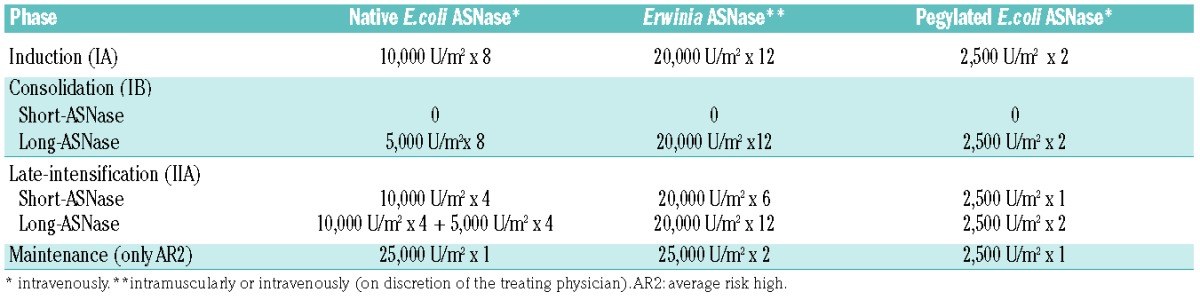
All non-very high-risk patients in complete remission or good partial response at the end of induction were randomly assigned to receive either conventional native E. coli ASNase therapy (12 doses, short-ASNase) or prolonged native E. coli ASNase therapy (24 doses, long-ASNase) (Figure 1). Patients in the short-ASNase arm had to receive 8×10,000 U/m2 in induction (IA) and 4×10,000 U/m2 in late intensification (IIA). The patients in the long-ASNase arm had to receive 12 extra doses, 8×5,000 U/m2 native E.coli ASNase in consolidation (IB) and 4×5,000 U/m2 extra doses in IIA. All native E.coli ASNase doses were scheduled twice a week. Patients with grade ≥2 allergy to native E. coli ASNase were switched to equivalent doses of Erwinia ASNase: four doses of native E. coli ASNase (5,000 or 10,000 U/m2) were replaced by six doses of 20,000 U/m2 Erwinia ASNase (3 per week, in view of the shorter half-life). As Erwinia ASNase was not available in Europe from the end of 2002 until 2006, patients were, in that time period, switched to equivalent doses of pegylated E. coli ASNase, and six doses of 20,000 U/m2 Erwinia ASNase in 2 weeks substituted by one dose of 2,500 U/m2 of pegylated E. coli ASNase (Table 1).
Table 1.
Native E.coli asparaginase administrations in different treatment cycles and switch to Erwinia or pegylated E.coli asparaginase in case of grade 2 or more allergy.
Definitions
Definitions of central nervous system disease and complete remission have been published previously12,13 and are summarized in the Online Supplementary Appendix. Treatment-related toxicity was graded according to the National Cancer Institute (NCI) Common Toxicity Criteria version 1994.15
Statistical analysis
The primary endpoint of this study was disease-free survival, secondary endpoints were overall survival and toxicity. In order to detect an increase in the 5-year disease-free survival rate from 84% (short-ASNase arm) to 89% (long-ASNase arm), corresponding to a treatment hazard ratio (HR) of 0.67, 1,500 patients had to be randomized, of whom 212 had to be followed until an event (log-rank test, 2-sided alpha=5%, power=80%).
Further information on endpoint definitions, randomization technique, stratification factors, and the statistical analysis16 is included in the Online Supplementary Appendix.
Results
Patients’ characteristics
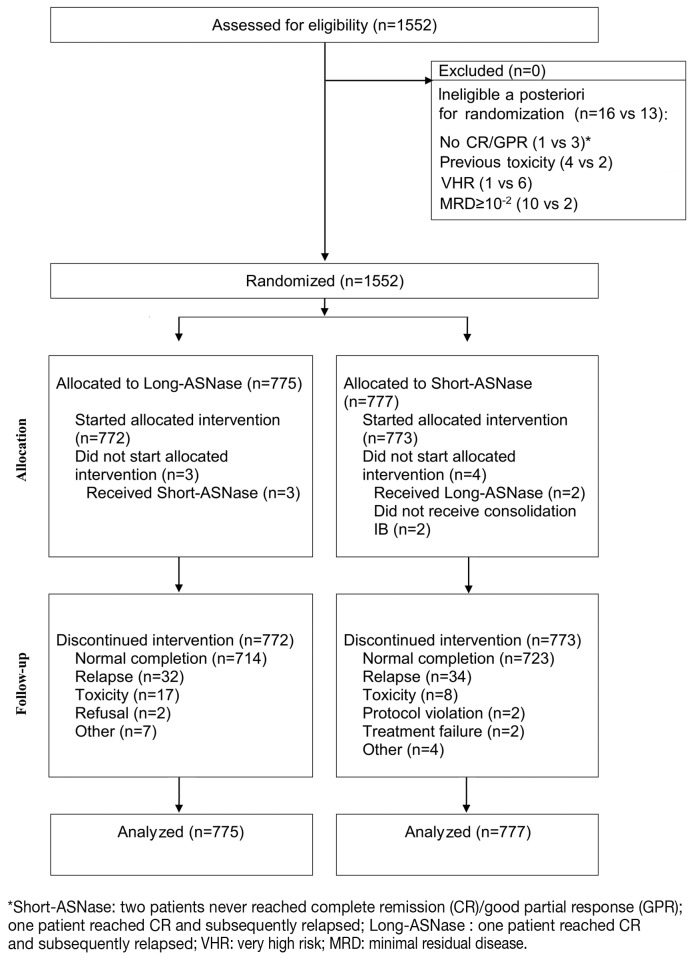
Between December 1998 and August 2008, 1,552 non-very high-risk patients (1,481 with ALL and 71 with NHL) were randomized in the EORTC-CLG trial 58951 to receive either long-ASNase (n=775) or short-ASNase (n=777). Of those patients 14.7% were very low risk, 66.2% average risk low and 19.1% average risk high (Table 2). Twenty-nine (16 long-ASNase and 13 short-ASNase) patients were considered a posteriori as being ineligible after randomization17,18 (Figure 2). The reasons for ineligibility were: no complete remission/good partial response (1 long-ASNase versus 3 short-ASNase), previous toxicity (4 versus 2), very high risk (1 versus 6), minimal residual disease ≥10−2 (10 versus 2). Nevertheless, those patients were included in the disease-free survival and overall survival analyses according to the intent-to-treat principle.
Table 2.
Patient and disease characteristics according to the native E. coli ASNase randomization.

Figure 2.
CONSORT statement in flow diagram.
The baseline characteristics of the patients and leukemia, first randomization, response to prephase and induction were evenly distributed in both treatment groups (Table 2).
Treatment applicability
Among the 775 patients allocated to the long-ASNase group, three patients never got the extra doses for unknown reasons and received the short-ASNase arm. In 58 patients the long-ASNase was interrupted for various reasons: relapse in 32, excessive toxicity in 17, parental refusal in two, and other reasons in seven patients.
Of the 777 patients allocated to the short-ASNase group, two patients erroneously received the long-ASNase treatment, and two did not receive the consolidation block. In 50 patients ASNase was interrupted prematurely: two because of failure to achieve remission, 34 because of relapse, eight because of excessive toxicity, two because of protocol violation and four for other reasons (Figure 2).
A total of 1,391 patients (641 treated with long-ASNase and 750 with short-ASNase) received the total number of administrations according to the protocol and randomization arm. In the long-ASNase group, 103 (13.3%) patients received an incomplete number of administrations and/or insufficient dose of ASNase, while 13 (1.7%) of the 777 patients in the short-ASNase arm were undertreated. Most of the protocol violations were due to a substitution with insufficient amount and/or doses of Erwinia ASNase (58 in the long-ASNase arm and 7 in the short-ASNase arm) after allergic reactions to native E. coli ASNase.
Treatment results
At a 7-year median follow-up there were 97 events in the long-ASNase arm and 111 in the short-ASNase group (Table 3). The two patients in the short-ASNase group who failed to achieve complete remission were considered as having had events at time 0. Relapse occurred in 87 children (11%) in the long-ASNase arm and in 102 children (13%) in the short-ASNase arm. The site of relapse for the long-ASNase versus short-ASNase comparison was isolated bone marrow (54 versus 60), combined bone marrow (16 versus 23), isolated central nervous system (10 versus 11) and other isolated relapse (7 versus 8). Death in complete remission occurred in ten children in the long-ASNase arm and in seven children in the short-ASNase arm. In the long-ASNase arm, three patients died of organ toxicity, four due to an infection, one due to graft-versus-host disease after hematopoietic stem cell transplantation for a secondary myelodysplasia, one patient died of a pre-existing cardiomyopathy during treatment and there was one unexplained, sudden death during late intensification.
Table 3.
Outcome, type of event, and toxicity according to the randomized arm.
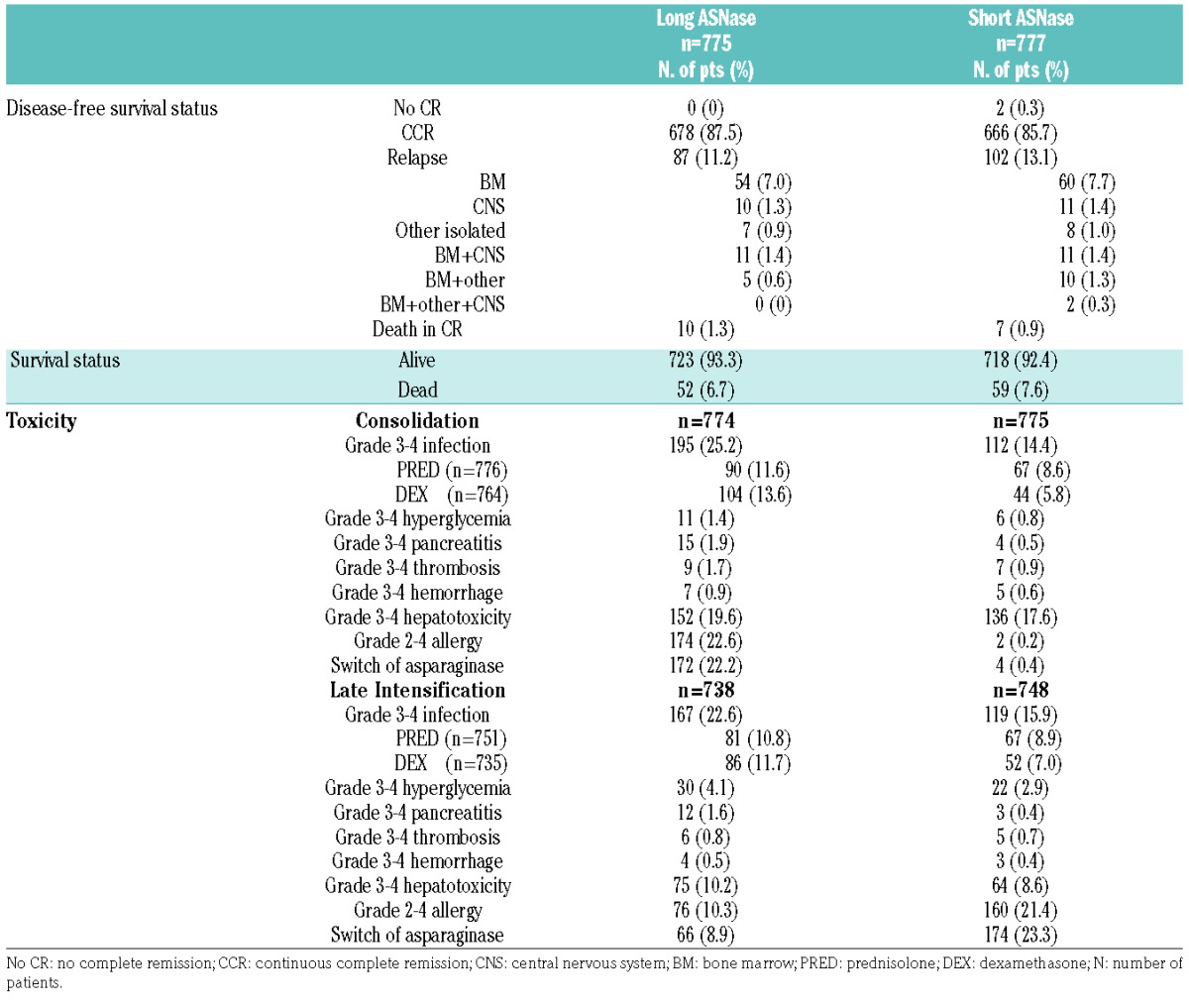
In the short-ASNase arm, one patient died due to organ toxicity, two due to an infection, two due to a secondary malignancy (1 central nervous system tumor and 1 medulloblastoma), one due to graft failure after hematopoietic stem cell transplantation for a secondary acute myeloid leukemia and one patient died of a pre-existing cardiomyopathy during treatment.
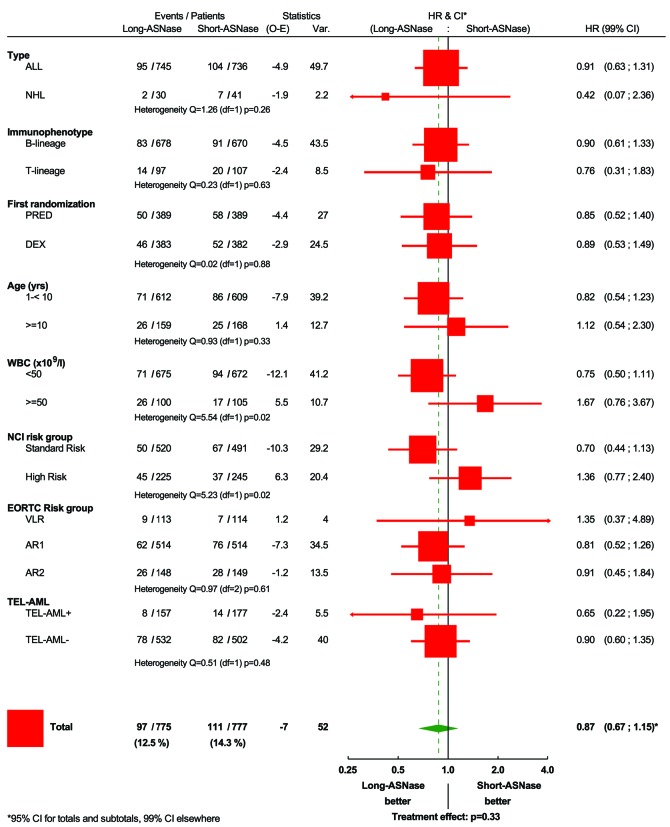
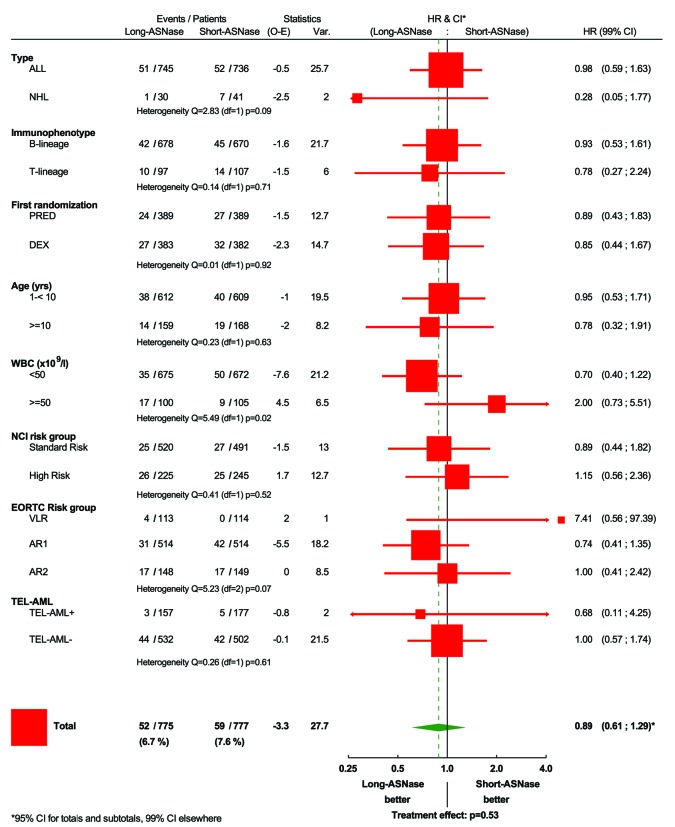
The 8-year disease-free survival rate (± standard error) was 87.0±1.3% in the long-ASNase and 84.4±1.4% in short-ASNase group (HR: 0.87; P=0.33) (Figure 3A). The 8-year overall survival rate was comparable in the two treatment arms: 92.6±1.0% in the long-ASNase group and 91.3±1.2% in the short-ASNase group (HR: 0.89; P=0.53) (Figure 3B). Subgroup analyses according to immunophenotype, EORTC risk group and the steroid assigned during induction (prednisolone versus dexamethasone) did not reveal significant heterogeneity in the treatment differences regarding disease-free survival and overall survival (Figures 4 and 5, Online Supplementary Figures S1–S3). In contrast, in the NCI standard-risk ALL group, the long-ASNase treatment had a positive effect [8-year disease-free survival: 89.7% (long-ASNase) versus 85.0% (short-ASNase), HR: 0.70; 99% confidence interval (CI): 0.44–1.13; P=0.057] (Figure 4, Online Supplementary Figure S4A), whereas in the NCI high-risk ALL group, a trend in the opposite direction was observed (HR: 1.36; 99% CI: 0.77–2.41; P=0.17) (Online Supplementary Figure S4C) (test for heterogeneity: P=0.02). The favorable effect of long-ASNase in this subgroup of patients was due to a decreased number of bone marrow relapses (34 isolated or combined bone marrow relapses versus 53 in the short-ASNase group). The incidence of isolated central nervous system (4 versus 5) and other isolated relapses (5 versus 6) was equally low in both arms. In contrast, regarding overall survival, no treatment difference was noted in NCI standard-risk (HR: 0.89) and high-risk ALL patients (Figure 5, Online Supplementary Figure S4B,D) (test for heterogeneity: P=0.52).
Figure 3.
(A) Disease-free survival and (B) overall survival according to the randomized arm.
Figure 4.
Forest plot regarding disease-free survival according to the randomization arm. ALL: acute lymphoblastic leukemia; NHL: non-Hodgkin lymphoma; VL: very low risk; AR1: average risk low; AR2: average risk high; DEX: dexamethasone; PRED: prednisolone; WBC: white blood cell count; NCI: National Cancer Institute; HR: hazard ratio; CI: confidence interval. *NCI Standard Risk: ALL with WBC <50×109/L and age 1 – <10 years; NCI High Risk: ALL with WBC ≥50×109/L or age <1 or age ≥10 years.
Figure 5.
Forest plot regarding overall survival according to the randomization arm. ALL: acute lymphoblastic leukemia; NHL: non-Hodgkin lymphoma; VLR: very low risk; AR1: average risk low; AR2: average risk high; DEX: dexamethasone; PRED: prednisolone; WBC: white blood cell count; NCI: National Cancer Institute; HR: hazard ratio; CI: confidence interval. *NCI Standard Risk: ALL with WBC <50×109/L and age 1 – <10 years; NCI High Risk: ALL with WBC ≥50×109/L or age <1 or age ≥10 years.
Restraining the treatment comparisons to eligible patients only (n=1,523), in particular to those receiving ASNase treatment according to the protocol guidelines (n=1,391) and to those patients without grade 2–4 ASNase allergy (n=1,154), similar results were obtained regarding disease-free survival and overall survival (data not shown).
Toxicity
The incidence of grade 3–4 infection was higher in the long-ASNase group than in the short-ASNase group during consolidation (25.2% versus 14.4%) and late intensification (22.6% versus 15.9%). This difference was more pronounced in patients who were randomly assigned to dexamethasone in induction (Table 3).
In both arms the incidence of grade 3–4 pancreatitis was low (<2%) with a trend to a slightly higher incidence in the long-ASNase group during consolidation (15 versus 4) and late intensification (12 versus 3). Grade 2–4 allergy to ASNase in the long-ASNase versus short-ASNase group was 22.6% versus 0.2% during the consolidation phase and 10.3% versus 21.4% in late intensification phase. In the long-ASNase group, the incidence of grade 2–4 allergic reactions was higher during consolidation (22.1%) than in late intensification (10.3%). The type of corticosteroid used in induction (prednisolone or dexamethasone) had no impact on the incidence of allergy grade during consolidation or late intensification. During the whole treatment period, the incidence of grade 2–4 allergy was 30.5% in the long-ASNase arm and 21.7% in the short-ASNase arm.
The incidences of other grade 3–4 toxicities reported during consolidation and late intensification were similar in the two randomization groups (Table 3).
Discussion
The prognosis of acute lymphoblastic malignancies has been improved steadily by the use of multidrug chemotherapy of which one of the key elements is ASNase. Several contemporary collaborative studies have demonstrated that intensification of ASNase therapy could offer an advantage in terms of outcome for the patients. Nevertheless, extrapolation of these findings is difficult as different dosages and sources of ASNase were applied in different chemotherapy backbones. This was the reason why the EORTC-CLG study group decided to investigate the effect of extended native E. coli ASNase therapy in consolidation and late intensification on top of a BFM-backbone for all non-very high risk patients as one of the randomized issues in the 58951 study.
We observed a slightly higher 8-year disease-free survival in the long-ASNase group (87.0% versus 84.4%; HR: 0.87; P=0.33) and a similar overall survival (92.6% versus 91.3%; HR: 0.89; P=0.53) rate in the long-ASNase group compared to the short-ASNase group. The analogous outcome rates were confirmed in subgroup analysis according to immunophenotype, EORTC risk group, and prednisolone/dexamethasone randomization. Exploratory analysis with an a posteriori classification of the ALL patients according to NCI risk criteria showed a trend towards higher 8-year disease-free survival rate for the long-ASNase patients in the NCI standard-risk group due to a lower number of bone marrow relapses. However, the 8-year overall survival rate was comparable in the two groups. Excluding patients who did not receive the ASNase treatment as planned according to the allocated arm or excluding allergic patients did not affect the disease-free survival rates. Our data are in accordance with the results of the AIEOP ALL-91 study, in which no advantage could be demonstrated for intermediate-risk patients randomized to receive either standard treatment (4×10,000 IU/m2 ASNase during late intensification) or weekly high-dose ASNase 25,000 IU/m2 for 20 weeks during late intensification and early continuation.9 The International BFM study group showed in the IDH-ALL-90 trial that extended high-dose native E. coli ASNase (25,000 IU/m2 weekly for 20 doses, starting from the beginning of continuation therapy) versus no ASNase could improve outcome in standard-risk ALL patients treated with a reduced-intensity BFM-based protocol. In this protocol no consolidation block IB was given and two doses of anthracyclines were omitted in late intensification.5 Pession et al.5 postulated that the benefit obtained from the extended native ASNase therapy in this study probably overcame the reduced leukemia control by the applied treatment reduction.
As in most BFM-based studies at that time, all EORTC-CLG patients received classical doses of 10,000 IU/m2 of native E. coli ASNase. For the additional doses in the long-ASNase arm, patients received 5,000 IU/m2 of native E. coli ASNase. The dose reduction was mainly dictated by the fear of an excess in toxicity for those patients. Ahlke et al. studied the pharmacokinetic and pharmacodynamic properties of different dosages of native E. coli ASNase. They found that 96% of the patients had complete asparagine depletion and sufficient native E. coli ASNase activity with a dose of 5,000 IU/m2 every third day.19 This observation gave sufficient evidence to apply a lower dosage of the additional doses of native E. coli ASNase during consolidation and late intensification in the long-ASNase group. As previously reported by Domenech et al.,12 the number of infections during induction was similar for the patients treated with dexamethasone or prednisolone in the EORTC-CLG 58951 trial. The concern of an excess in toxicity due to extended native E. coli ASNase use turned out to be correct as patients in the long-ASNase arm had a trend towards more grade 3–4 infections during consolidation and late intensification, especially when they received dexamethasone in induction. Others have reported that dexamethasone was associated with a higher risk of infection during consolidation treatment, particularly in older children and adolescents.10,20 The excess in severe infections during consolidation and late intensification could be the result of the additional myelosuppressive effect of prolonged administration of native E. coli ASNase in combination with intensive treatment10,21 and decreased immunoglobulin production resulting from decreased protein synthesis. Although moderate, hematologic toxicity from prolonged ASNase treatment might increase the immunosuppressive effect of dexamethasone, especially due to the fact that ASNase diminishes dexamethasone clearance.22
As expected due to the longer exposure to the drug, the cumulative incidence of grade 2–4 allergy was higher in the long-ASNase arm than in the short-ASNase arm (30.5% versus 21.7%). Although intermittent administration of native E. coli ASNase is considered to be a risk factor for allergy, our study demonstrated that the rate of grade 2–4 allergic reactions in the long-ASNase arm was higher in consolidation than in late intensification (22.1% versus 10.3%). Concomitant administration of corticosteroids in late intensification reduces the risk of clinical hypersensitivity reactions, whereas the protective effect of the corticosteroids is absent in consolidation.2,23 Although concurrent administration of corticosteroids reduces the clinical signs of allergic reactions, it does not influence the neutralizing antibodies and the subsequent inactivity of ASNase. It is known that not only the frequency of allergy, but also the incidence of silent inactivation increases with longer duration of native E. coli ASNase exposure. As native E. coli ASNase activity monitoring was not performed in the EORTC 58951 trial, the incidence of silent inactivation in this study remains unclear. However, it is likely that the long treatment arm had more silent inactivators who subsequently received insufficient ASNase in the late intensification phase. The EORTC 58951 study ran in a period in which therapeutic drug monitoring was not routinely performed to detect silent inactivation and subtherapeutic asparaginase activity. Nowadays it is well known that therapeutic drug monitoring is essential in order to optimize asparaginase treatment and therefore an increasing number of study groups are including such monitoring in their study protocols.
Despite the fact that grade 3–4 pancreatitis was rare in both treatment arms (<2%), there was a trend to a slightly higher incidence among patients randomized to the long-ASNase group. The incidences of other typical ASNase-related grade 3–4 toxicities, such as hyperglycemia, thrombosis, hemorrhage and hepatotoxicity, were similar in the two treatment groups.
Two large, randomized studies3,4 showed superiority of native E. coli ASNase over Erwinia ASNase when given at the same dose and frequency. In the EORTC-CLG trial 58881, 700 patients were randomized to receive either native E. coli or Erwinia ASNase at a dosage of 10,000 IU/m2 twice weekly during induction and late intensification. The patients randomized to receive Erwinia ASNase had lower 6-year event-free survival (59.8% versus 73.4%; P=0.0004) and overall survival (75.1% versus 83.9%; P=0.002) rates than those randomized to native E. coli ASNase.3 In the DFCI 95-01 trial, 286 standard and high-risk patients received either 25,000 IU/m2 weekly of native E. coli or Erwinia ASNase. Patients treated with Erwinia ASNase had an inferior 10-year event-free survival rate (75.2% versus 84.6%; P=0.02) and overall survival rate (85.3% versus 93.1%; P=0.04).4 In both studies, patients treated with Erwinia ASNase experienced less toxicities but more relapses,3,4 Although all patients in the EORTC-CLG 58951 study received native E. coli ASNase as first-line treatment, a large proportion of the patients in the long-ASNase arm had to be switched to Erwinia ASNase due to allergic reactions. Based on the results of the EORTC-CLG 58881 study, the subsequent 58951 trial recommended higher dosages of Erwinia ASNase (20,000 IU/m2 3 times a week) for patients with an allergic reaction. Nevertheless, despite these dose recommendations 7.5% of patients in the long-ASNase group erroneously received insufficient doses of Erwinia ASNase, which was only the case in 0.9% of the patients assigned to short-ASNase treatment.
As already mentioned, at the start of the study, therapeutic drug monitoring of ASNase was not common practice in childhood ALL protocols. An increasing number of study groups now incorporate the monitoring of ASNase activity to optimize treatment efficacy. It is widely accepted that ASNase activity is optimal if trough activity levels are >100U/L.2 A recent DCOG study highlighted the short activity of Erwinia ASNase by measuring trough levels of ASNase activity. Effective ASNase activity levels were found in 100% (at 48 h) and 33% (at 72 h) of patients treated with 20,000 IU Erwinia ASNase twice or three times a week.24 In our study, allergic patients received Erwinia ASNase three times a week, mostly on Monday, Wednesday and Friday. Although ASNase monitoring was not routinely performed in our study, extrapolating the data of Tong et al. we assume that at least some of our patients receiving Erwinia ASNase were undertreated during the weekend. 24This, together with the fact that fewer patients in the long-ASNase arm received all intended doses as planned in the protocol, could mitigate the potential benefit of prolonged ASNase treatment. Moreover, prolonged native E. coli ASNase therapy resulted in an increase in allergic reactions, pancreatitis and infections which can hamper the treatment efficacy by omission or delay of other essential chemotherapeutics.
It is important to note that ASNase therapy in our study consisted of treatment for 12 weeks in the long-ASNase arm versus 6 weeks in the short arm, given in a discontinuous way. Our extended arm is not comparable with the very prolonged and continuous use of ASNase (30 weeks) in the DCOG and DFCI protocols. In the DCOG ALL10 protocol, patients with medium risk according to minimal residual disease received therapy intensification including 30 weeks of PEG-ASNase exposure and dexamethasone/vincristine pulses. This resulted in a significantly higher 5-year event-free survival rate compared with that in historical controls (88% versus 76%; P=0.056).25 An increase in event-free survival was also observed in the DFCI ALL 91-01 trial for patients who tolerated prolonged high-dose native E. coli ASNase (25,000 IU/m2 weekly) or PEG-ASNase (2,500 IU/m2 every other week) for 30 weeks compared to patients who tolerated less than 20 weeks of extended ASNase therapy.7 The relatively short extension of ASNase treatment in our trial together with the increased risk of silent inactivation and allergy due to the discontinuous administrations, may have nullified the effect seen in the very prolonged, continuous schedules of the DCOG and DFCI protocols. Moreover, we used native E. coli ASNase, which is being increasingly replaced by PEG-ASNase in contemporary childhood ALL trials, due to the longer action and the lower incidence of allergy and silent inactivation.
In conclusion, this study demonstrates that a 6-week prolongation of native E. coli ASNase therapy in a discontinuous administration schedule (during consolidation and late intensification) does not significantly improve patients’ overall outcome. Our study underscores the hypothesis of Pession that the overall treatment intensity of this BFM-based regimen, with four-drug induction for all patients, already results in maximal therapeutic efficacy, whereas intensification with prolonged native E. coli ASNase therapy does not add any benefit for these patients.5 One has to be cautious to extrapolate our results to other studies especially if less intensive induction therapy, other asparaginase preparations or other administration schedules are used. Future studies should aim to optimize the efficacy of ASNase therapy, not only by prolonged administration but by the use of PEG-ASNase and by therapeutic drug monitoring in real time in order to promptly intercept silent inactivation and subtherapeutic asparaginase activity.
Supplementary Material
Acknowledgments
The authors would like to thank the EORTC-CLG study group members for their participation in the study and the EORTC HQ Data Management Department members (Séraphine Rossi, Lies Meirlaen, Liv Meert, Aurélie Dubois, Christine Waterkeyn, Alessandra Busato, Isabel VandeVelde and Gabriel Solbu) for their support in this trial as well as Drs. Francisco Bautista (EORTC-CLG fellow) and Matthias Karrasch (former EORTC Clinical Research Physician).
A complete list of the members of the Children's Leukemia Group of the European Organization for Research and Treatment of Cancer appears in the Online Supplementary Data.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/10/1727
References
- 1.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J Clin Oncol. 2015;33(27):2938–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avramis VI, Tiwari PN. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int J Nanomedicine. 2006; 1(3):241–254. [PMC free article] [PubMed] [Google Scholar]
- 3.Duval M, Suciu S, Ferster A, et al. Comparison of Escherichia coli-asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trial. Blood. 2002;99(8):2734–2739. [DOI] [PubMed] [Google Scholar]
- 4.Moghrabi A, Levy DE, Asselin B, et al. Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood. 2007;109(3):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pession A, Valsecchi MG, Masera G, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005; 23(28):7161–7167. [DOI] [PubMed] [Google Scholar]
- 6.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999; 13(3):335–342. [DOI] [PubMed] [Google Scholar]
- 7.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–1218. [DOI] [PubMed] [Google Scholar]
- 8.Vilmer E, Suciu S, Ferster A, et al. Long-term results of three randomized trials (58831, 58832, 58881) in childhood acute lymphoblastic leukemia: a CLCG-EORTC report. Leukemia. 2000;14(12):2257–2266. [DOI] [PubMed] [Google Scholar]
- 9.Rizzari C, Zucchetti M, Conter V, et al. L-asparagine depletion and L-asparaginase activity in children with acute lymphoblastic leukemia receiving i.m. or i.v. Erwinia C. or E.coli L-asparaginase as first exposure. Ann Oncol. 2000;11(2):189–193. [DOI] [PubMed] [Google Scholar]
- 10.Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Critical Rev Oncol Hematol. 1998;28(2):97–113. [DOI] [PubMed] [Google Scholar]
- 11.Mondelaers V, Suciu S, De Moerloose B, et al. Prolonged E. Coli asparaginase therapy does not improve significantly the outcome for children with low and average risk acute lymphoblastic leukemia (ALL) and non hodgkin lymphoma (NHL): final report of the EORTC-CLG randomized phase III trial 58951 [abstract]. Blood 2012;120:134. [Google Scholar]
- 12.Domenech C, Suciu S, De Moerloose B, et al. Dexamethasone (6 mg/m2/day) and prednisolone (60 mg/m2/day) were equally effective as induction therapy for childhood acute lymphoblastic leukemia in the EORTC CLG 58951 randomized trial. Haematologica. 2014;99(7):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Moerloose B, Suciu S, Bertrand Y, et al. Improved outcome with pulses of vincristine and corticosteroids in continuation therapy of children with average risk acute lymphoblastic leukemia (ALL) and lymphoblastic non-Hodgkin lymphoma (NHL): report of the EORTC randomized phase 3 trial 58951. Blood. 2010;116(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavé H, Van Der Werff Ten Bosch J, Suciu S, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia. New Engl J Med. 1998;339(9):591–598. [DOI] [PubMed] [Google Scholar]
- 15.https://www.eortc.be/services/doc/ctc/
- 16.Kalbfleisch JD, Prentice RL. The Clinical Analysis of Failure Time Date. 2nd ed. Hoboken NJ: Wiley InterScience; 2002. [Google Scholar]
- 17.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11:32.20334632 [Google Scholar]
- 18.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trial. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahlke E, Nowak-Gottl U, Schulze-Westhoff P, et al. Dose reduction of asparaginase under pharmacokinetic and pharmacodynamic control during induction therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 1997;96(4):675–681. [DOI] [PubMed] [Google Scholar]
- 20.Vrooman LM, Stevenson KE, Supko JG, et al. Consolidation dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: Results from a randomized study–Dana-Farber Cancer Institute ALL Consortium Protocol 00–01. J Clin Oncol. 2013;31(9):1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merryman R, Stevenson KE, Gostic WJ, 2nd, et al. Asparaginase-associated myelosuppression and effects on dosing of other chemotherapeutic agents in childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;59(5):925–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawedia JD, Liu C, Pei D, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012; 119(7):1658–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong WH, Pieters R, de Groot-Kruseman HA, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014; 99(11):1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34(22):2591–2601. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.