Abstract
Background
Coronary heart disease (CHD) is the primary cause of death in individuals with chronic kidney disease (CKD), but current equations for assessing coronary risk have low accuracy in this group. We have reported that the addition of a genetic risk score (GRS) to the Framingham risk function improved its predictive capacity in the general population. The aims of this study were to evaluate the association between this GRS and coronary events in the CKD population and to determine whether the addition of the GRS to coronary risk prediction functions improves the estimation of coronary risk at the earliest possible stages of kidney disease.
Methods
A total of 632 CKD patients, aged 35–74 years, who had Stage 4–5 CKD, were on dialysis, had a functioning renal transplant or had returned to dialysis after transplant failure were included and followed up for a mean of 9.3 years. The transitions between disease states and the development of coronary events were registered. The increase in predictive ability that was obtained by including the GRS was measured as the improvement in the C-statistic and as the net reclassification index.
Results
The GRS was independently associated with the risk of CHD (hazards ratio 1.34; 95% confidence interval 1.04–1.71; P = 0.022), especially in Stages 4 and 5 CKD, and kidney transplant patients. A coronary risk prediction function that incorporated chronic kidney disease (CKD) disease state, age, sex and the GRS had significantly greater predictive capacity (AUC 70.1, P = 0.01) and showed good reclassification (net reclassification improvement 28.6).
Conclusion
This new function, combining genetic and clinical data, identifies CKD patients with a high risk of coronary events more accurately, allowing us to prevent such events more effectively.
Keywords: cardiovascular risk, chronic kidney disease, genetic risk score, kidney transplant, prevention
Introduction
Chronic kidney disease (CKD) is a public health problem worldwide due to its high prevalence [1] and associated morbidity and mortality [2, 3]. CKD is characterized by the progression toward terminal uraemia, requiring treatment with dialysis or kidney transplantation [4].
CKD patients experience 15–30 times greater age-adjusted cardiovascular disease (CVD) mortality compared with the general population [3, 4]. CVD mortality in individuals who are on haemodialysis (HD) is high—20% annually [5]. This risk declines following kidney transplantation but remains 3–5 times higher than in the general population [6, 7].
Measures to prevent coronary heart disease (CHD) aim at lowering risk factor levels in those who are at the highest risk. CHD risk estimation systems are used to make logical management decisions and help avoid under- and overtreatment. CHD risk in the general population is assessed using classical risk functions, such as the Framingham risk score. However, these tools fail to identify high-risk individuals accurately among CKD patients [8, 9], perhaps because traditional risk factors alone do not account for the morbidity and mortality that are associated with CHD in CKD patients [9]. Several attempts to develop a disease-specific risk score for CHD risk stratification in CKD have been made, but none has attained widespread clinical use [10–12].
We have reported an association between CHD and a multilocus genetic risk score (GRS), based on genetic variants that are associated with CHD but not classical cardiovascular risk factors (CVRFs) [13]. We have also shown that this GRS improves the predictive capacity of the Framingham risk function in the general population [13] in terms of discrimination and reclassification.
The aims of the current study were to examine the association between our coronary GRS and coronary events in individuals with CKD and determine whether the inclusion of this GRS improves the predictive capacity of CHD risk tools at the earliest stages of kidney disease.
Materials and methods
Patient sample, follow-up and phenotype definition
This prospective and observational study included 632 CKD patients, aged 35–74 years, who had Stage 4 or 5 CKD (CKD 4/5 group), were receiving renal replacement therapy by HD or peritoneal dialysis (PD) (dialysis group), had a functioning renal transplant (transplant group) or had returned to dialysis after renal transplantation (transplant failure group); all patients had been followed up for over 3 years by the nephrology service at Marques de Valdecilla University Hospital.
The transitions between defined disease states (CKD 4/5, dialysis, transplantation and transplant failure) and the development of coronary events [fatal or non-fatal myocardial infarction (MI), angina pectoris and coronary revascularization] were recorded for 620 patients during the follow-up. The identification of these events was based on in-person attendance at the clinic and on revision of the patients’ medical records. MI was defined per American Heart Association/American College of Cardiology (AHA/ACC) criteria [14], angina was based on the presence of typical symptoms and an objective demonstration of myocardial ischaemia (electrocardiography, coronary stenosis, etc.), and percutaneous coronary intervention and coronary artery by-pass grafting were considered to be revascularization procedures. Death due to CHD was determined following a review of the mortality register—when the most likely cause of death was CHD and no other cause could be ascribed.
Demographic and clinical parameters were also recorded, including age, sex, race, height, weight, stage of renal disease, disease aetiology, type of dialysis prior to transplant (HD or PD), time in dialysis, previous transplants and the date of transplantation and classical coronary risk factors (smoking history, hypertension, hypercholesterolaemia and diabetes).
Biochemical parameters were measured during the first visit to the nephrology department for CKD: total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, creatinine levels, glycaemia, glycated haemoglobin (HbA1c), calcium, phosphorus, intact parathyroid hormone (iPTH), albumin and 24-h proteinuria. Systolic and diastolic blood pressure was also recorded. Renal function was estimated using the modified Modification of Diet in Renal Disease (MDRD) equation [15].
This study was performed by the nephrology service of Marqués de Valdecilla University Hospital (Santander, Spain), and the study protocol was approved by the institutional review board of the same hospital. Written informed consent was obtained from all subjects, and all clinical research was conducted per the Declaration of Helsinki and the 2008 Istanbul Declaration.
Genotyping and construction of the multilocus risk score
DNA samples were extracted from the patients’ blood using standard methods and genotyped by Gendiag.exe (Esplugues de Llobregat, Spain) using the Cardio inCode Score array (Ferrer inCode, Barcelona, Spain), which is based on the Veracode (Illumina, San Diego, CA, USA) and KASPar (KBioscience, Hoddesdon, UK) technologies [16]. The overall percentage of agreement between the chip and reference technology was 99.9%, and the analytical sensitivity and specificity exceeded 98.6%.
Genetic variants that are associated with CHD but not CVRFs (blood pressure, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, diabetes, smoking) were selected, and a weighted multilocus GRS was constructed as described [13]. The variants that we selected were: rs10455872 in LPA, rs12526453 in PHACTR1, rs1333049 near CDKN2A/2B, rs17465637 in MIA3, rs501120 in CXCL12, rs6725887 in WDR12, rs9818870 in MRAS, rs9982601 near SCL5A3 and haplotype B of ALOX5AP, which comprises rs10507391, rs17222842 and rs931505. The weight for each variant was based on the individual estimated effect size in the Coronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) study [17].
Estimation of 10-year coronary risk
Coronary risk was estimated using the modified Framingham risk function for Spain Registre Gironí del COR (REGICOR) [18]. This function includes age, sex, systolic and diastolic blood pressure, total cholesterol, HDL cholesterol, smoking status and diabetes status; the GRS was added to the function as published [13], where indicated.
We derived three additional risk estimation models by Cox regression analysis: Model 1 included age, sex and disease state; Model 2 further incorporated HDL cholesterol, hypercholesterolaemia, diabetes, HbA1c and hypertension; and Model 3 added MDRD, calcium, phosphorus, iPTH and albumin.
Statistical analysis
We used standard parametric and non-parametric methods to compare the characteristics of the CKD sample. We tested for associations between the incidence of coronary events and the GRS [as a continuous variable in standard deviation (SD) units] using Cox proportional hazards models, with adjustments for CVRFs. The transition of disease states from CKD 4/5 to dialysis, transplantation and transplant failure was considered a time-dependent variable in the Cox regression models.
We used two statistics to assess the potential value of including the GRS in a risk prediction model:
Improvements in the discriminatory capacity of the model were evaluated, based on the change in the C-statistic [19]
Improvements in reclassification were calculated using the net reclassification improvement (NRI) index [20]. To this end, we defined four risk categories (low, intermediate-low, intermediate-high and high), with cut-off points that were defined according to current guidelines (REGICOR: 0–5%, 5–10%, 10–15%, ≥15%) [13]. We calculated the expected number of events at 10 years in each risk category using Kaplan–Meier estimates [21] and used a bootstrapping method to construct the confidence intervals for the NRI to account for uncertainty in the Kaplan–Meier estimates, as recommended by Steyerberg and Pencina [21]
All analyses were performed using the R statistical package (version 2.11) [22].
Results
Sample characteristics, renal disease and coronary events
Our cohort included 632 CKD patients (205 females and 427 males), with a median follow-up of 9.3 years and mean age of 53.4 years. We detected 73 coronary events during the follow-up. In terms of progression of disease state, at the initial stage (inclusion into the study), 220 individuals had CKD 4/5 (34.8%), 161 were undergoing dialysis (25.5%, 27 PD and 134 HD), 200 had undergone a kidney transplantation (31.6%) and 51 had experienced transplant failure (8.1%). Table 1 shows the rates of disease progression during the study period from the initial stage in the 620 patients from whom we collected clinical data.
Table 1.
Transition between disease states in patients during the study period
| Disease Stage |
Final stage |
(Number of patients) |
||
|---|---|---|---|---|
| Initial stage | Number of patients | Dialysis | Transplant | Transplant failure |
| CKD 4/5 | 220 | 112 | 82 | 26 |
| Dialysis | 161 | 40 | 102 | 19 |
| Transplant | 200 | 0 | 162 | 38 |
| Transplant failure | 51 | 0 | 0 | 51 |
| Total | 632 | 152 | 346 | 134 |
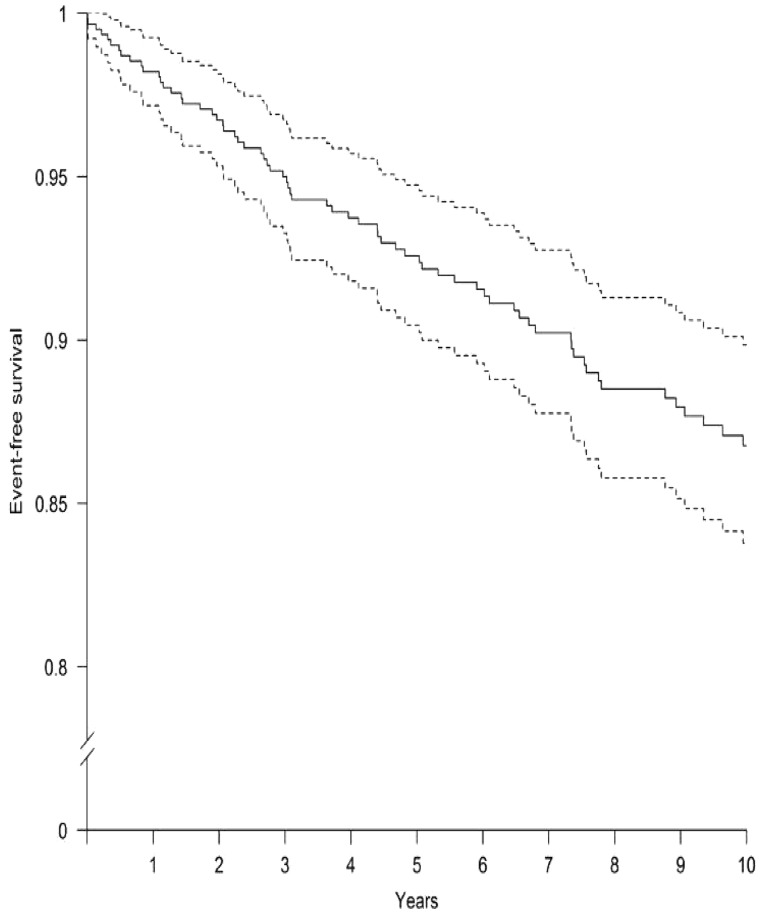
Compared with the general Spanish population [23], CKD patients had a higher 10-year incidence of coronary events (4.9% versus 13.2%, respectively; P < 0.001). Figure 1 shows the incidence of coronary events of the study patients over time by Kaplan–Meier analysis.
Fig. 1.
The incidence of the coronary events along the time using the Kaplan–Meier method. Event-free survival time with 95% CI. Solid line represents the mean, and the dotted lines represent 95% CIs.
Table 2 shows the characteristics of this CKD sample. The proportions of males, smokers, patients with a family history of CVD and those with CKD 4/5 were higher in individuals who had had a coronary event during the follow-up—these subjects also had generally lower HDL cholesterol and iPTH levels.
Table 2.
Characteristics of the CKD study population according to the incidence of coronary events
| Variables | No CHD events | CHD events | |
|---|---|---|---|
| (n = 547) | (n = 73) | p-value | |
| Age, n (SD) | 53.0 (10.7) | 55.3 (10.4) | 0.074 |
| Female, n (%) | 190 (34.7) | 13 (17.8) | 0.006 |
| Male, n (%) | 357 (65.3) | 60 (82.2) | 0.006 |
| Smoking status, n (%) | 0.080 | ||
| No | 102 (58.6) | 12 (37.5) | |
| Yes | 55 (31.6) | 15 (46.9) | |
| Ex-smoker | 17 (9.77) | 5 (15.6) | |
| Total cholesterol, mmol/L (SD) | 5,4 (1.2) | 5.32 (1.5) | 0.690 |
| LDL cholesterol, mmol/L (SD) | 3.26 (1.03) | 3.15 (1.00) | 0.451 |
| HDL cholesterol, mmol/L (SD) | 1.39 (0.26) | 1.20 (0.43) | 0.001 |
| Systolic blood pressure, mmHg (SD) | 141 (20.1) | 140 (20.8) | 0.955 |
| Diastolic blood pressure, mmHg (SD) | 82.2 (12) | 82.6 (10.7) | 0.808 |
| Diabetes, n (%) | 108 (19.7) | 18 (24.7) | 0.409 |
| Hypertension, n (%) | 463 (84.6) | 61 (83.6) | 0.946 |
| Glycaemia, mg/dL (95% CI) | 5.61 (5.11–5.88) | 5.66 (5.05–6.6) | 0.898 |
| HbA1c, % (95% CI) | 5.60 (5.20–6.30) | 5.80 (5.35–6.60) | 0.149 |
| Family history, n (%) | 91 (16.6) | 41 (56.2) | <0.001 |
| Disease states, n (%) | |||
| CKD 4/5 | 182 (33.3) | 38 (52.1) | 0.002 |
| Dialysis | 141 (25.8) | 15 (20.5) | 0.402 |
| Transplant | 179 (32.7) | 16 (21.9) | 0.083 |
| Transplant failure | 45 (8.23) | 4 (5.48) | 0.557 |
| MDRD, mL/min/1.73 m2 (95% CI) | 19.2 (8.75–43.6) | 20.5 (10.5–32.9) | 0.911 |
| Creatinine, μmol/L (95% CI) | 274 (141–557) | 291 (186–504) | 0.811 |
| Calcium, mmol/L (95% CI) | 2.31 (2.1–2.44) | 2.24 (2.1–2.4) | 0.127 |
| Phosphorus, mmol/L (95% CI) | 1.36 (1.09–1.81) | 1.39 (1.09–1.74) | 0.927 |
| iPTH, pmol/L (95% CI) | 16.55 (7.64–32.57) | 23.98 (10.06–41.59) | 0.019 |
| Albumin, μmol/L (95% CI) | 5.94 (5.51–6.38) | 5.94 (5.36–6.38) | 0.791 |
| Protein, urine, g/24 h | 0.67 (0.10–2.55) | 0.82 (0.23–2.37) | 0.142 |
| GRS-1, arbitrary unitsa (95% CI) | −0.19 (−0.83 to 0.72) | −0.01 (−0.621 to 1.02) | 0.088 |
| GRS-2, arbitrary unitsa, b (95% CI) | −0.07 (−0.70 to 0.67) | 0.29 (−0.24 to 0.84) | 0.028 |
GRS with standardized values (mean=0, typical deviation=1)
Analysed only in 597 individuals.
Association between GRS and coronary events in CKD patients
The results of the multivariate-adjusted association analysis between the time to CHD event and the clinical variables and GRS are shown in Table 3. Age, male gender, smoking (current or former) and levels of phosphorus and iPTH correlated directly with CHD events in our CKD sample. In contrast, calcium, albumin, eGFR and kidney transplantation were inversely associated with CHD events.
Table 3.
Bivariate association between clinical variables, genetic risk score and time to coronary heart events
| Variables | HR | 95% CI | P-value |
|---|---|---|---|
| Age | 1.42 | 1.14–1.76 | 0.002 |
| Male | 2.42 | 1.33–4.42 | 0.004 |
| Smoking status | |||
| No | Ref | Ref | Ref |
| Yes | 2.28 | 1.07–4.87 | 0.034 |
| Ex-smoker | 2.27 | 0.80–6.46 | 0.123 |
| Total cholesterol | 0.93 | 0.88–0.97 | 0.002 |
| LDL cholesterol | 0.93 | 0.88–0.99 | 0.028 |
| HDL cholesterol | 0.72 | 0.61–0.85 | <0.001 |
| Systolic blood pressure | 1.03 | 0.92–1.16 | 0.567 |
| Diastolic blood pressure | 0.87 | 0.71–1.06 | 0.159 |
| Diabetes | 1.53 | 0.91–2.59 | 0.110 |
| Hypertension | 1.78 | 0.72–4.41 | 0.215 |
| Dyslipidaemia | 1.58 | 0.99–2.49 | 0.056 |
| Glycaemia | 1.01 | 0.98–1.04 | 0.416 |
| HbA1c | 1.09 | 0.91–1.29 | 0.358 |
| Disease states | |||
| CKD 4/5 | Ref | Ref | Ref |
| Dialysis | 1.10 | 0.52–2.30 | 0.803 |
| Transplant | 0.25 | 0.11–0.55 | 0.001 |
| Transplant failure | 0.47 | 0.19–1.14 | 0.094 |
| MDRD | 0.88 | 0.79–0.99 | 0.029 |
| Creatinine | 1.04 | 0.97–1.11 | 0.270 |
| Calcium | 0.82 | 0.67–0.99 | 0.041 |
| Phosphorus | 1.18 | 1.05–1.32 | 0.004 |
| iPTH | 1.01 | 1.00–1.01 | 0.003 |
| Albumin | 0.48 | 0.32–0.71 | <0.001 |
| Protein, urine | 0.94 | 0.86–1.02 | 0.134 |
| GRS-1 (per SD unit) | 1.25 | 1.00–1.56 | 0.048 |
| GRS-2 (per SD unit) | 1.34 | 1.04–1.71 | 0.022 |
The GRS (hereafter referred to as GRS-1) had a linear relationship with the incidence of CHD [hazards ratio (HR) per SD unit 1.25; 95% confidence interval (CI) 1.00–1.56; P = 0.048]. However, several variants in this GRS had missing value rates of >9% (rs10455872, rs1050739, rs17222842 and rs9315051) (Supplementary Table S1). Thus, a second GRS (GRS-2) was constructed, removing these variants. GRS-2 also correlated linearly with the incidence of CHD (HR 1.34; 95% CI 1.04–1.71; P = 0.022).
We detected a statistically significant interaction between these GRSs and disease state, prompting us to stratify the analysis by disease state (Table 4). GRS-2 was associated with coronary events in patients with CKD-4/5 and transplant patients. GRS-1 was also linked to coronary events in CKD 4/5 patients.
Table 4.
Association of GRSs (as a continuous variable in SD units) with coronary events by disease state
| GRS |
GRS-1 |
GRS-2 |
||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age and sex-adjusted | ||||
| CKD 4/5 | 3.0 (1.71–5.26) | <0.001 | 2.05 (1.14–3.7) | 0.017 |
| Dialysis | 1.03 (0.67–1.57) | 0.899 | 0.92 (0.57–1.49) | 0.739 |
| Transplant | 1.26 (0.88–1.8) | 0.210 | 1.64 (1.10–2.45) | 0.016 |
| Transplant failure | 0.88 (0.44–1.75) | 0.711 | 1.12 (0.61–2.07) | 0.713 |
| Adjusted by estimated coronary risk according to the REGICOR function | ||||
| CKD 4/5 | 2.86 (1.83–4.46) | <0.001 | 2.33 (1.38–3.92) | 0.002 |
| Dialysis | 1.12 (0.66–1.92) | 0.672 | 0.87 (0.49–1.52) | 0.618 |
| Transplant | 1.17 (0.88–1.58) | 0.315 | 1.43 (1.03–1.99) | 0.033 |
| Transplant failure | 0.90 (0.42–1.91) | 0.779 | 1.23 (0.63–2.43) | 0.544 |
For GRS composition, see Supplementary Table S1.
Predictive capacity of the risk functions: discrimination and reclassification
We analysed the predictive capacity of four CHD risk functions: the Framingham tool, adapted to the Spanish population (the REGICOR function), and three functions that were developed in this study, incorporating the association between clinical variables and CHD events (Table 2). This analysis was performed with and without the inclusion of GRSs (Table 5). Inclusion of the GRSs into REGICOR significantly improved its predictive capacity (C-statistic: GRS-1 66.8, P = 0.027; GRS-2 66.0, P = 0.04 compared with 63.5 for REGICOR alone) (Table 5, part A).
Table 5.
Analyses of improvement in predictive capacity: (A) discrimination of the REGICOR function when the GRSs were included; (B) changes in discrimination and reclassification when GRS-2 was included in the REGICOR risk function, considering only patients with CKD 4/5 or a kidney transplant; (C) changes in discrimination and reclassification when GRS-2 was included in the new model risk functions
| |||||||
|
REGICOR |
REGICOR + GRS-1 |
P-value |
REGICOR + GRS-2 |
P-value |
|||
| C-Statistic (95% CI) | 63.5 (56.4–70.6) | 66.8 (59.7–73.8) | 0.027 | 66.0 (59.1–72.9) | 0.044 | ||
|
NRI (95% CI) |
−2.5 (−16.8 to 11.8) |
2.3 (−19.5 to 24.0) |
|||||
| |||||||
|
REGICOR |
REGICOR + GRS-2 |
P-value |
|||||
| C-Statistic (95% CI) | 52.5 (41.3–63.6) | 60.1 (49.6–70.7) | 0.019 | ||||
|
NRI (95% CI) |
22.8 (−10.0 to 55.5) |
||||||
| (C) New predictive models based on age, sex, disease state, and clinical and biochemical variables in patients with CKD 4/5 or a kidney transplant | |||||||
|
REGICOR |
MODEL 1 |
MODEL 1 + GRS-2 |
MODEL 2 |
MODEL 2 + GRS-2 |
MODEL 3 |
MODEL 3 + GRS-2 |
|
| C-Statistic (95% CI) | 52.5 (41.3–63.3) | 62.9 (52.6–73.3) P-value = 0.001 | 70.1 (61.3–78.8) P-value = 0.01 | 65.3 (54.7–75.9) | 69.6 (60.3–78.9) P-value = 0.05 | 69.5 (59.5–79.4) | 73.7 (64.1–83.4) P-value = 0.06 |
| NRI (95% CI) | 28.6 (11.8–45.4) | 25.5 (6.3–44.7) | 23.4 (−8.1 to 54.9) | ||||
Model 1: age, sex, disease state; Model 2: Model 1 + HDL cholesterol, dyslipidaemia, diabetes, HbA1c, hypertension; Model 3: Model 2 + MDRD, calcium, phosphorus, iPTH, albumin.
Because the association between the GRSs and CHD events was only significant in patients with CKD 4/5 or a kidney transplant, we analysed the predictive capacity of the CHD risk functions with and without the GRS in these patient groups. Adding GRS-2 significantly improved the predictive ability of REGICOR with regard to discrimination [C-statistic: 60.1 (49.6–70.7) versus 52.5 1 (41.3–63.6), P = 0.019, for REGICOR alone], but not reclassification [NRI = 22.8% (−10.0 to 55.5)] (Table 5, part B).
The predictive capacity of the newly developed CHD risk functions was better than that of REGICOR. CHD risk function-1, which included age, sex and disease state (CKD 4/5 or transplant), had a C-statistic of 62.9 (95% CI 52.6–73.3), which increased further on incorporation of GRS-2 [C-statistic 70.1 (61.3–78.8), P = 0.013] (Table 5, part C). Moreover, CHD risk function-1 with GRS-2 showed significant improvement with regard to reclassification [NRI = 28.6% (11.8–45.4)] (Table 5, part C). The predictive capacity of CHD risk function-2 and CHD risk function-3 was also significantly higher than that of REGICOR alone, which improved by adding GRS-2, yielding a significant improvement in discrimination and reclassification for CHD risk function 2 + GRS-2 versus CHD risk function 2 and a non-significant improvement for CHD risk function 3 + GRS-2 versus CHD risk function 3 (Table 5, part C).
Discussion
Current recommendations on the prevention of coronary disease in clinical practice stress the need for interventions that are based on an assessment of the individual’s global risk [24, 25]. Several risk-scoring systems, such as the Framingham risk function, have been developed to determine an individual’s global CHD risk. However, current CHD risk equations for the general population fail to accurately identify high-risk individuals among CKD patients [8]. There has been extensive research into the value of new biomarkers in identifying patients with an increased risk of cardiovascular events in the general population and in patients with CKD. An accurate assessment of cardiovascular risk at an early stage would facilitate earlier, more aggressive and focused treatment in persons with a greater need for preventive measures—ultimately to reduce event rates. Several such markers have emerged, although their use in routine clinical applications has yet to be fully established.
Attempts to develop a disease-specific risk score for cardiac risk stratification in CKD have also been made [10–12], most of which have been focused on renal transplant candidates, but none has achieved widespread clinical use. In contrast, we evaluated the association between our GRS and coronary events in the CKD population and determined whether its addition to coronary risk prediction tools improves coronary risk estimations at the earliest possible stage of kidney disease—not merely at the transplant stage.
The risk of developing CHD depends on several lifestyle and genetic factors. Further, heritable factors account for as much as 60% of the variation in risk [26]. Genome-wide association study (GWAs) have identified several tag single nucleotide polymorphisms (SNPs) in various loci throughout the genome that are robustly associated with CHD. When combined in a GRS—especially with clinical variables—these SNPs become useful in predicting CHD events [27]. We used this approach in a previous study [13] and in this work. Prompted by the observation that our genetic panel improves the predictive capacity of risk functions for the general population [13], we examined whether this benefit could be obtained in CKD patients, a population in whom CHD events must be predicted more accurately.
Our data show that the inclusion of genetic variants significantly improves the predictive capacity of the modified Framingham risk function for Spain (REGICOR) in CKD patients, effecting a rise in discrimination capacity and a non-significant increase in reclassification. Moreover, a simpler function that incorporates disease state, age, sex and our GRS for CKD 4/5 and kidney transplant patients yields better results. Our function provides 10-year prediction rates, applicable to CKD 4/5 and renal transplant patients, and improves the predictive capacity of classical coronary risk functions in a direct comparison with a very good reclassification index.
Clinically, this new CHD risk function—combining disease state, age, sex and our GRS—by identifying CKD patients who are at risk more accurately, would facilitate earlier, more aggressive, and focused preventive treatment of CKD 4/5 and transplanted CKD patients, lowering the rates of clinically relevant coronary events. CKD patients who are at high risk, according to our novel tool, would be candidates for statin therapy, based on their efficacy in decreasing LDL cholesterol levels and global CHD risk. Statins have been proven to be effective for primary prevention of CVD in CKD patients [28].
Strengths and limitations
We highlight the following strengths of our study. We included a population of CKD patients who were followed up for a mean of 9.3 years, from kidney failure to dialysis, transplantation and transplant failure, which allowed us to evaluate the robustness of the GRS. Also, the variants that were included in our score represent loci that have actual relevance to CHD risk and are independent of CVRFs [13]; thus, our GRS provides complementary information to that provided by classical risk functions. Finally, this GRS improves classical risk functions and allows us to adapt them to the CKD population in the form of three new predictive models, meriting further study.
The main limitations of this study are the size of the population and the limited number of events that was observed. Also, because this study was a single-centre report, replication of the results is needed.
A relative limitation is that the missing individuals for some of the variants accounted for the differences between GRS-1 and GRS-2. When GRS-1 was analysed on removal of the individuals with missing values, the performance of GRS-1 and GRS-2 was identical (data not shown).
That we used a limited number of genetic variants could be considered a limitation. However, in the general population, the inclusion of more variants did not improve the results that were obtained with the GRSs that were used in this study [29].
Although the GRS is not associated with cardiovascular events/deaths in patients who are undergoing dialysis, this is not a limitation. Arrhythmias, but not coronary disease, have been shown to be responsible for 65% of all cardiac deaths, or 27% of all-cause mortality, in HD patients [30]. We have confirmed the strong association of the QTc interval and other clinical data with mortality in CKD patients who are on HD [31].
In this study, we have characterized the association between our multilocus GRS [13] and the incidence of CHD events in a CKD cohort. Moreover, we found that a simple function that combines age, sex and disease state with the GRS predicts coronary events in CKD patients better, allowing strategies to prevent the development of coronary events to be implemented.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals
Funding
Supplementary Material
Acknowledgments
This work was supported in part by the Spanish Ministry of Science and Innovation within the National Plan for Scientific Research, Technological Development and Innovation 2008–2011 and was co-financed by the European Regional Development Fund (ERDF), ‘Prediction of cardiovascular risk in patients with chronic kidney disease’ (IPT-010000-47).
References
- 1. Zhang Q-L, Rothenbacher D.. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dekker FW, De Jager DJ, Vervloet MG. et al. Noncardiovascular mortality in CKD: an epidemiological perspective. Nat Rev Nephrol 2014; 10: 208–214 [DOI] [PubMed] [Google Scholar]
- 3. de Jager DJ, Grootendorst DC, Jager KJ. et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009; 302: 1782–1789 [DOI] [PubMed] [Google Scholar]
- 4. Ross L, Banerjee D.. Cardiovascular complications of chronic kidney disease. Int J Clin Pract 2013; 67: 4–5 [DOI] [PubMed] [Google Scholar]
- 5. Bradbury BD, Fissell RB, Albert JM. et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2007; 2: 89–99 [DOI] [PubMed] [Google Scholar]
- 6. Parajuli S, Clark DF, Djamali A.. Is kidney transplantation a better state of CKD? Impact on diagnosis and management. Adv Chronic Kidney Dis 2016; 23: 287–294 [DOI] [PubMed] [Google Scholar]
- 7. Jardine AG, Gaston RS, Fellstrom BC. et al. Prevention of cardiovascular disease in adult recipients of kidney transplants. Lancet 2011; 378: 1419–1427 [DOI] [PubMed] [Google Scholar]
- 8. Weiner DE, Tighiouart H, Elsayed EF. et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol 2007; 50: 217–224 [DOI] [PubMed] [Google Scholar]
- 9. Silver SA, Huang M, Nash MM. et al. Framingham risk score and novel cardiovascular risk factors underpredict major adverse cardiac events in kidney transplant recipients. Transplantation 2011; 92: 183–189 [DOI] [PubMed] [Google Scholar]
- 10. Armstrong KA, Rakhit DJ, Case C. et al. Derivation and validation of a disease-specific risk score for cardiac risk stratification in chronic kidney disease. Nephrol Dial Transplant 2005; 20: 2097–2104 [DOI] [PubMed] [Google Scholar]
- 11. Lewis MS, Wilson RA, Walker KW. et al. Validation of an algorithm for predicting cardiac events in renal transplant candidates. Am J Cardiol 2002; 89: 847–850 [DOI] [PubMed] [Google Scholar]
- 12. Gowdak LH, de Paula FJ, César RL. et al. A new risk score model to predict the presence of significant coronary artery disease in renal transplant candidates. Transplant Res 2013; 2: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lluis-Ganella C, Subirana I, Lucas G. et al. Assessment of the value of a genetic risk score in improving the estimation of coronary risk. Atherosclerosis 2012; 222: 456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaffe AS. Third universal definition of myocardial infarction. Clin Biochem 2013; 46: 1–4 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Bosch JP, Lewis JB. et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470 [DOI] [PubMed] [Google Scholar]
- 16. Semagn K, Babu R, Hearne S. et al. Single nucleotide polymorphism genotyping using Kompetitive Allele Specific PCR (KASP): overview of the technology and its application in crop improvement. Mol Breeding 2014; 33: 1–14 [Google Scholar]
- 17. Schunkert H, König IR, Kathiresan S. et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat Genet 2011; 43: 333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marrugat J, Agostino RD, Sullivan L. et al. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J Epidemiol Community Heal 2003; 57: 634–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newson R. Confidence intervals for rank statistics: Somers’D and extensions. Stata J 2006; 6: 309–334 [Google Scholar]
- 20. Pencina MJ, D’Agostino RB, Steyerberg EW.. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011; 30: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steyerberg EW, Pencina MJ.. Reclassification calculations for persons with incomplete follow-up. Ann Intern Med 2010; 152: 195–197 [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team. R: The R Project for Statistical Computing. http://www.r-project.org/ (20 May 2015, date last accessed)
- 23. Marrugat J, Solanas P, D’Agostino R. et al. [Coronary risk estimation in Spain using a calibrated Framingham function]. Rev española Cardiol 2003; 56: 253–261 [DOI] [PubMed] [Google Scholar]
- 24. Perk J, De Backer G, Gohlke H. et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J 2012; 33: 1635–1701 [DOI] [PubMed] [Google Scholar]
- 25. Goff DC, Lloyd-Jones DM, Bennett G. et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines . Circulation 2014; 129 (25 Suppl 2): S49–S73 [DOI] [PubMed] [Google Scholar]
- 26. Sayols-Baixeras S, Lluís-Ganella C, Lucas G. et al. Pathogenesis of coronary artery disease: focus on genetic risk factors and identification of genetic variants. Appl Clin Genet 2014; 7: 15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Musunuru K, Hickey KT, Al-Khatib SM. et al. Basic concepts and potential applications of genetics and genomics for cardiovascular and stroke clinicians: a scientific statement from the American Heart Association. Circ Cardiovasc Genet 2015; 8: 216–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Major RW, Cheung CK, Gray LJ. et al. Statins and cardiovascular primary prevention in ckd: a meta-analysis. Clin J Am Soc Nephrol 2015; 10: 732–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iribarren C, Lu M, Jorgenson E. et al. Clinical utility of multi-marker genetic risk scores for prediction of incident coronary heart disease: a cohort study among over 51 thousand individuals of European ancestry. Circ Cardiovasc Genet 2016; doi:10.1161/CIRCGENETICS.116.001522 [DOI] [PubMed] [Google Scholar]
- 30. Lullo L Di, Rivera R, Barbera V. et al. Sudden cardiac death and chronic kidney disease : From pathophysiology to treatment strategies. Int J Cardiol 2016; 217: 16–27 [DOI] [PubMed] [Google Scholar]
- 31. Genovesi S, Rossi E, Nava M. et al. A case series of chronic haemodialysis patients: mortality, sudden death, and QT interval. Europace 2013; 15: 1025–1033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.