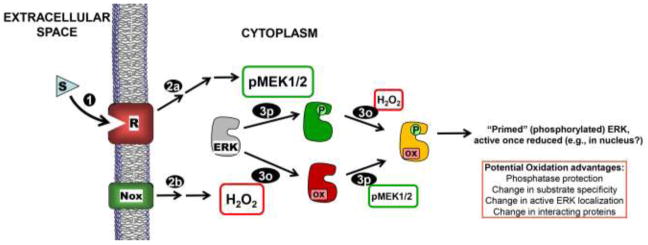
Figure 7.
Model for ERK phosphorylation and oxidation controlling activity in response to extracellular signals. Receptor (R) activation due to stimulus (S) binding (1) leads to MEK1/2 activation (2p) as well as concurrent NADPH oxidase (Nox) activation to produce H2O2 (2o). Activated MEK1/2 phosphorylates inactive ERK1/2 (gray or red) on Thr and Tyr residues (3p), and ERK1/2 is reversibly oxidized by H2O2 or a derivative thereof (3o) (our data suggest that neither modification precludes the other). Our data support a strong inhibitory effect of this oxidation on phospho-ERK. Note that dually phosphorylated AND oxidized ERK (yellow) is “primed” to be active once reduced (e.g., by thioredoxin or other dithiol). Note that this diagram does not emphasize the high degree of localization that is expected to be important in the posttranslational modification of ERK due to signal-mediated receptor activation.