Abstract
A 45-year-old man had developed a choroidal neovascular membrane (CNVM) in his left eye at the age of 38 years and had received six intravitreal ranibizumab injections with resulting visual acuities of 6/60 in the affected eye and 6/4 in the unaffected right eye (Snellen charts). Family history and genetic testing revealed tissue inhibitor of metalloproteinase-3 (TIMP3) gene positive Sorsby fundus dystrophy (SFD). The patient has been under regular follow-up since. At the age of 45 years, he presented with subretinal fluid accumulation in his right eye suggestive of CNVM and received six intravitreal ranibizumab injections, which maintained visual acuity of 6/7.5 in his right eye. Although SFD is a rare condition, it should be suspected and ruled out in young patients presenting with suspicious fundoscopic findings and subretinal fluid on optical coherence tomography. Early intervention can possibly delay macular fibrosis and loss of vision secondary to SFD associated with CNVM.
Keywords: eye, retina, genetics
Background
Sorsby fundus dystrophy (SFD) is a rare, late-onset disease characterised by the loss of central vision. Its manifestations include drusen-like deposits, choroidal neovascularisation in the majority of cases and, occasionally, atrophy of the peripheral choroid.1 Mutations in the tissue inhibitor of metalloproteinase-3 (TIMP3) gene have been implicated in SFD. However, SDF is often misdiagnosed due to an overlap in clinical symptoms with other types of maculopathies or macular dystrophies and a lack of information on additional family members who may be affected by the disease.2 Recently, the intravitreal administration of anti-vascular endothelial growth factor (anti-VEGF) agents was reported to improve clinical outcomes in patients with SFD who develop a choroidal neovascular membrane (CNVM).3–5
Case presentation
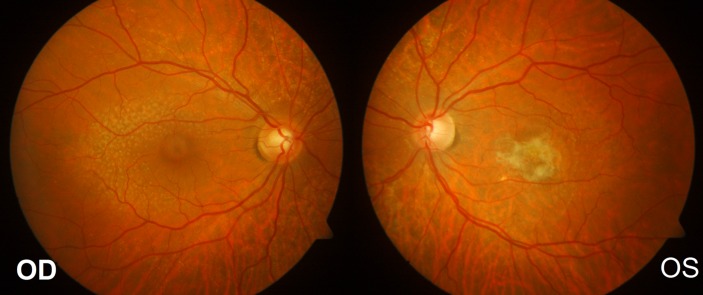
A 45-year-old man had presented at the age of 38 years to a different ophthalmology unit with a slight reduction of central vision in his left eye. According to his medical record, funduscopy of the left eye revealed small drusen within the temporal arcade. The optical coherence tomography (OCT) findings were suggestive of subretinal fluid under the left macula, and the patient was initially diagnosed with central serous retinopathy based on these findings. As the condition failed to resolve spontaneously, a fluorescein angiogram was performed and confirmed a left subretinal neovascular membrane. Subsequently, he was treated with three intravitreal injections of ranibizumab (Lucentis 0.5 mg/0.05 mL; Genentech, California, USA) followed by another three injections administered on a pro re nata basis over the subsequent months. The visual acuities after this treatment were 6/60 in the left eye secondary to macular fibrosis and 6/4 in the right eye (figure 1).
Figure 1.
Fundus images at presentation. (A)Right eye fundus image revealing small drusen within the temporal arcade.(B) Left eye fundus image revealing central macular fibrosis.
Investigations
A detailed family history taken after the treatment revealed central vision loss in the patient's deceased father, paternal uncle and brother. Genetic tests revealed a pathogenic variant of the TIMP3 gene (heterozygous positive state for c.610A>T in exon 5). Given these results, the patient was advised that he could face similar issues with his second eye and to present early if any new symptoms occur.
Differential diagnosis
Central serous chorioretinopathy which was suspected by the previous clinicians.
Other dominantly inherited macular dystrophy, such as vitelliform macular dystrophy, and other macular or pattern dystrophies which may also present with CNVM, for example, Best and PRPH2 mutations.
Treatment
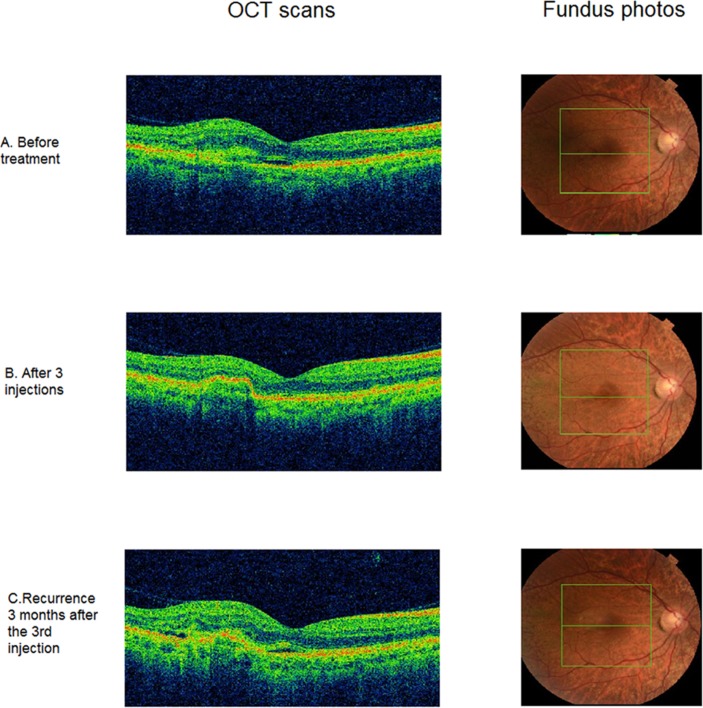
Clinically, the patient remained stable over the course of the next 5 years. He presented in early 2016 to the emergency department of the Leicester Royal Infirmary, complaining of subtle visual distortion in the right eye. He had a visual acuity of 6/5−2 in the right eye and hand movements in the left eye. Imaging with optical coherence tomography (Topcon 3D OCT; Topcon Corporation, Tokyo, Japan) showed early subretinal fluid in the right eye (figure 2A). A course of three loading intravitreal ranibizumab injections administered at monthly intervals was initiated within 10 days from presentation; this treatment maintained a visual acuity of 6/4 in the right eye at 3 months and had resolved the subretinal fluid accumulation by 4 months (figure 2B).
Figure 2.
Optical coherence tomography (Topcon 3D OCT) of the right eye (RE) findings during the course of treatment and follow-up. (A) OCT and fundus photos (RE) before treatment. (B) OCT and fundus photo (RE) after three intravitreal injections where there is no exudation and minimal fibrosis is observed. (C) Recurrence of the disease with the right eye OCT showing minimal exudation but significant fibrosis temporally to the fovea.
Outcome and follow-up
At 6 months following the emergency presentation and 4 months after the third injection, the subretinal fluid increased in volume, requiring two further monthly ranibizumab injections, which were administered on a pro re nata regimen (figure 2C). Visual acuity on his recent clinic visit was 6/7.5 in the right eye and hand movements in the left eye.
Discussion
The initial deferred treatment led to reduced visual outcome in the left eye secondary to macular fibrosis. Although the age of the patient and the OCT findings suggested central serous retinopathy, a fluorescein angiogram should always be considered by clinicians given the bilateral presence of drusen within the temporal arcades. A more detailed family history in such late-onset macular dystrophy is important and needs to be included in the clinical assessment. Our patient was able to provide quite a detailed family tree which would have helped direct the differentials towards the correct diagnosis at an early stage of the presentation.
On having confirmed SFD clinically and genetically, regular clinic follow-ups and patient education are warranted.
The response to ranibizumab has been excellent in our patient, as the subretinal fluid has resolved and the visual acuity maintained, which significantly altered the predicted clinical course of CNVM secondary to SFD. Our patient is undergoing regular follow-ups and is still being actively treated. Treatment involves four to six weekly follow-up visits. In clinic settings where the capacity is not available to offer such intensive follow-up regimens, patient education is crucial to detect and treat early subretinal neovascular membranes. Early detection of CNVM could potentially prevent severe loss of central visual acuity and a decrease in the quality of life. It may be interesting to assess different regimens for anti-VEGF agents such as fixed injections or even treat-and-extend protocols. However, given the small number of patients, this assessment is rather difficult.
Learning points.
Presence of subretinal fluid and/or subretinal fibrosis in patients of young age should be investigated with fluorescein angiograms to exclude choroidal neovascular membrane (CNVM) development.
Early suspicion of inherited macular disorders and prompt treatment should be encouraged to avoid long-term fibrosis and loss of vision.
Anti-vascular endothelial growth factor treatment is promising, but optimal regimens and dosages are yet to be defined in rare conditions such as Sorsby fundus dystrophy (SFD). The behaviour of CNVM appears to be different in such diseases from that in well-studied diseases such as age-related macular degeneration. Specifically, exudation is minimal, but the development of fibrosis appears to be rapid in SFD.
Footnotes
Contributors: NM monitored data collection, analysed the data and drafted the paper. SB contributed in data acquisition. TE analysed data and provided administration support. KTT critically revised the paper and he is the guarantor. All authors approved submission.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sorsby A, Mason ME, Gardener N. A fundus dystrophy with unusual features. Br J Ophthalmol 1949;33:67–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alapati A, Goetz K, Suk J, et al. . Molecular diagnostic testing by eyeGENE: analysis of patients with hereditary retinal dystrophy phenotypes involving central vision loss. Invest Ophthalmol Vis Sci 2014;55:5510–21. 10.1167/iovs.14-14359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaskas K, Hovan M, Mahmood S, et al. . Ranibizumab for the management of Sorsby fundus dystrophy. Eye 2013;27:101–2. 10.1038/eye.2012.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemenetzi MK, Luff AJ, Lotery AJ, et al. . Successful treatment of choroidal neovascularization secondary to sorsby fundus dystrophy with intravitreal bevacizumab. Retin Cases Brief Rep 2011;5:132–5. 10.1097/ICB.0b013e3181cc216b [DOI] [PubMed] [Google Scholar]
- 5.Keller J, Giralt J, Alforja S, et al. . Altering the clinical course of Sorsby fundus dystrophy with the use of anti-vascular endothelial growth factor intraocular therapy. Retin Cases Brief Rep 2015;9:104–5. 10.1097/ICB.0000000000000103 [DOI] [PubMed] [Google Scholar]