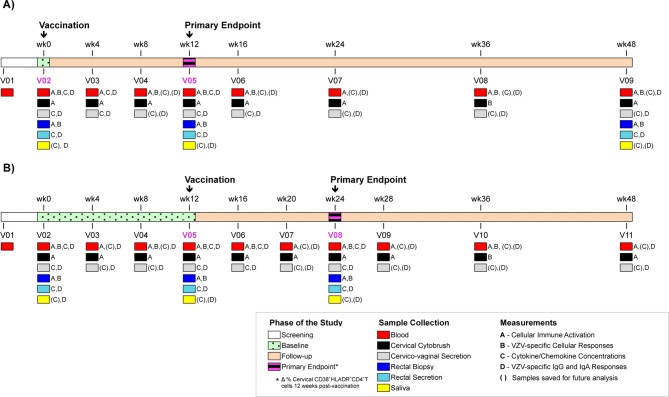
Figure 1.
Schematic representation of enrolment and follow-up for KAVI-VZV-001 trial. (A) Immediate group and (B) delayed group. Immediate group receive a single dose of VZVOka vaccine at week 0 and delayed group at week 12. Participants are followed-up for a period of 36–48 weeks after receiving the vaccine. VZV, varicella-zoster virus.