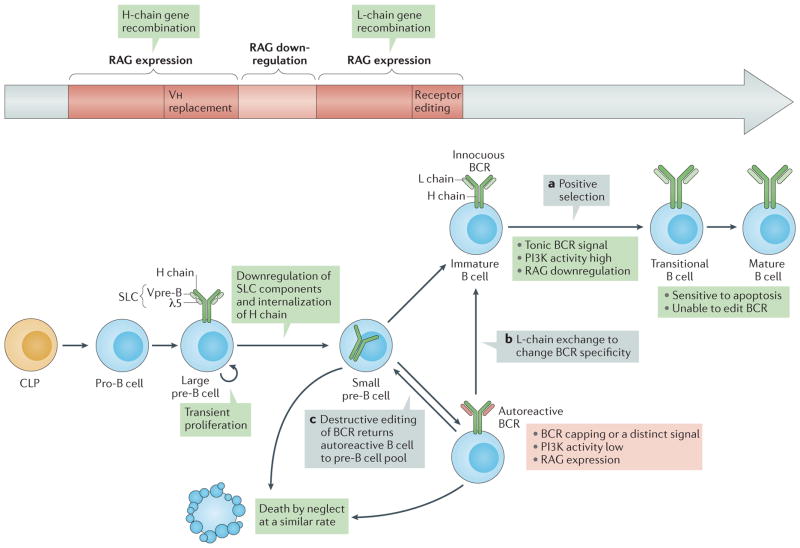
Figure 1. B cell development.
Pro-B cells in the bone marrow, which are derived from common lymphoid progenitor (CLP) cells, initiate heavy (H)-chain gene rearrangement through expression of recombination-activating genes (RAG1 and RAG2; collectively referred to here as RAG) and epigenetic modifications of the H-chain loci that promote accessibility6. Productive H-chain gene assembly leads to the association of IgM H-chain (μ-chain) protein with surrogate light-chain (SLC) components λ5 (also known as IGLL1) and Vpre-B, and surface expression of the pre-B cell receptor (pre-BCR) in large pre-B cells162,163. Pro-B cells can also undergo H-chain variable region (Vh) replacement reactions, whereby Vh elements recombine to a conserved heptamer within the Vh element of an already rearranged VDJ exon164. Spontaneous, antigen-independent triggering of the pre-BCR promotes progression to the large pre-B cell stage, which involves downregulation of RAG expression and transient proliferation. Differentiation to the small pre-B cell stage follows; at this stage, SLC components are downregulated, RAG is re-expressed and RAG activity is redirected to the L-chain genes163. L chains that pair with H chains trigger tonic BCR signalling, which promotes positive selection when the BCR is non-autoreactive (part a) or receptor editing (parts b and c) when the BCR is autoreactive or if tonic signalling is impaired. Editing can lead to exchange of one functional L chain for another, which can render the BCR innocuous and allow developmental progression (part b), or to secondary rearrangements that prevent L-chain expression (part c), such as out-of-frame joins that destroy the original L-chain gene but fail to replace it, which returns the cell to the pre-B cell compartment25,69. Cells that go through positive selection enter the transitional B cell stage, at which stage B cells seem to be extremely sensitive to apoptosis while losing the ability to edit the BCR97,98. Small pre-B cells and editing B cells have a similar turnover rate, which I interpret to indicate that they die ‘by neglect’ owing to prolonged insufficiency of phosphoinositide 3-kinase (PI3K)–AKT activity.