Abstract
Exposure to nitrogen dioxide (NO2), a by product of combustion, is associated with poor asthma control in children. We sought to determine whether gas-fueled kitchen appliance use is associated with 24-hour indoor NO2 concentrations and whether these concentrations are associated with asthma morbidity in children. Children aged 5–12 years old with asthma were eligible. Mean 24-hour NO2 concentration was measured in the kitchen over a four-day sampling period and gas stove use was captured in time activity diaries. The relationship between stove and oven use and daily NO2 concentration was analyzed. Longitudinal analysis assessed the effect of daily NO2 exposure on symptoms, inhaler use, and lung function. Multivariate models were adjusted for age, sex, season, and maternal education. Thirty children contributed 126 participant days of sampling. Mean indoor 24-hour NO2 concentration was 58(48) ppb with a median (range) of 45(12-276) ppb. All homes had gas stoves and furnaces. Each hour of kitchen appliance use was associated with an 18ppb increase in 24-hour NO2 concentration. In longitudinal multivariate analysis, each ten-fold increase in previous-day NO2 was associated with increased night time inhaler use (OR=4.9, p=0.04). There were no associations between NO2 and lung function or asthma symptoms. Higher previous-day 24-hour concentration of NO2 is associated with increased night time inhaler use in children with asthma.
Keywords: Asthma, household air pollution, nitrogen dioxide, gas stove
1. Introduction
Asthma affects nearly 25 million people in the United States, including 10% of children[1], and is the most common noncommunicable disease among children worldwide[2]. The current therapeutic strategy focuses on control of the illness through medications and avoidance of factors that exacerbate the disease, including allergens, tobacco smoke, irritants, and air pollution.[3] Long term exposure to elevated concentrations of nitrogen dioxide (NO2), a byproduct of consumption, may contribute to development of asthma [4]. Several epidemiologic studies have shown associations between exposure to NO2 and increased asthma symptoms, including chest tightness, shortness of breath, wheeze, and cough, an increase in rescue inhaler use, and an increased number of asthma attacks among children with established disease. [4–9] Because of the adverse health effects of NO2 exposure, in the United States, outdoor concentrations of NO2 are regulated by the EPA Clean Air Act [4], and the 2005 World Health Organization (WHO) air quality guidelines suggest limits for indoor NO2 concentrations worldwide[10].
The indoor residential environmental may contain several sources of NO2, and prior studies have shown that presence of a gas cooking appliance (gas stove and oven; herein referred to as gas stove) in the home is associated with higher NO2 concentrations.[6, 8, 11–13]. Across the United States, 35% of homes report using gas stoves, and use is particularly concentrated in urban areas[14]. These indoor sources of NO2 are used intermittently, resulting in highly fluctuating NO2 concentrations, and real-life studies quantifying the effect of stove use on indoor NO2 concentrations are limited. Furthermore, the multi-day averaging concentration data commonly used in health outcomes research often fail to reflect the short-term concentrations and temporal variability in exposures produced by indoor sources.[15, 16] Short-term increases in NO2 concentrations, including daily concentrations, may reflect independent risk to adverse respiratory outcomes, in addition to adverse effects noted with longer-term average exposures.[13, 17, 18] In this study, we investigate the relationship between use of gas stoves and 24-hour NO2 concentrations, and how daily NO2 concentration impacts asthma morbidity in a cohort of children living in Baltimore city, where gas stove use is common (83% in prior studies)[6].
2. Methods
2.1 Study design
Thirty participants living in homes with gas stoves were recruited from the ASTHMA-DIET study, a longitudinal cohort with the aim to examine how diet influences susceptibility to environmental exposures in children with asthma. Eligibility criteria included 1) Age 5–12 years, 2) Physician diagnosis of asthma, 3) Symptoms of asthma and/or reliever medication use in past 6 months, and 4) Residence in catchment area of East Baltimore. Exclusion criteria included a current diagnosis of another major pulmonary disease and planning to relocate residence during the study period. 24-hour NO2 monitoring occurred daily in the home over a period of 4–7 days during one of the three, seven-day monitoring periods part of ASTHMA-DIET. The Johns Hopkins Medical IRB approved the protocol and Informed consent was obtained from all individual participants included in the study.
2.2 Environmental assessment
Home inspection documenting type of stove and heat source along with general housing characteristics was conducted by a trained home inspector. Mean 24-hour NO2 concentrations were measured using passive samplers (Ogawa badge) in the kitchen. Badges typically were deployed from 4pm on Day 1 to 4pm on Day 2. Daily mean outdoor NO2 concentration data was obtained from publically available datasets.[19] Time activity diaries were filled out by the primary care giver to document whether combustion sources were used over three eight-hour time periods over the course of a day (any/no use during the eight-hour time period) and whether windows were opened in the home for greater than 10 minutes (yes/no). In a subset of willing participants (n=18), detailed time activity diaries captured gas stove use in 15-minute increments throughout the day.
2.3 Clinical assessment
Asthma symptom burden was assessed using the Pediatric Asthma Diary, a two-part, validated diary that accurately detects clinically important changes in asthma status over a 24-hour period. The daytime diary assesses the frequency of breathing problems, activity limitations, bother of daytime symptoms, report of absence from school, unscheduled visits to the doctor, emergency room or hospital along with the number of albuterol puffs used over the course of the day. The nighttime diary examines the number of awakenings caused by asthma symptoms on a 4-point scale of 0 (no awakenings) to 3 (awake all night) along with number of nighttime rescue inhaler puffs.[20] Twice-daily spirometry (FEV1) was assessed using the PiKo-1 Electronic Peak Flow/FEV1 Meter (PiKo-1, nSpire Health, Inc). The nighttime diary questions were answered in the morning shortly after the child awoke, and the daytime diary was completed in the afternoon/early evening prior to the child’s bedtime. Following instruction from research coordinators, caregivers completed the daily diary with input from children and supervised daily lung function recordings. Diaries were reviewed by research coordinators during the three home visits over the course of each study week.
2.4 Statistical analysis
Descriptive statistics were analyzed using Spearman correlations, chi square tests, t-tests, and Wilcoxon rank-sum tests, as appropriate. Linear regression models were used to estimate the relationship between duration of gas stove use and indoor 24-hour NO2 concentration; in multivariate analysis these models were adjusted for season (cold season November 1- March 31 based on typical home heating patterns in Baltimore city; non-cold season April 1-October 31, Baltimore Gas and Electric), outdoor NO2 concentration, and window opening. Effect modification was explored by including an interaction term of stove use * season to determine whether season modified the effect of kitchen appliance use on indoor 24-hour NO2 concentration, and a separate analysis included stove use * window opening to determine whether the relationship between stove use and NO2 concentration varied by window opening. We also created an interaction between stove use, season, and window opening to determine whether season and window use jointly influenced the relationship between duration of cooking and 24-hour indoor NO2 concentration.
To examine the role of NO2 exposure on asthma outcomes, as the distribution of mean 24-hour NO2 concentration was right skewed, log-transformed 24-hour NO2 concentration was used as the primary exposure variable in generalized estimating equations models[21] to account for repeated measures. Dichotomous yes/no variables were created to capture any report of breathing problems, activity limitations, bother, or nighttime awakenings over the course of the day. Inhaler use was defined as a dichotomous variable (no puffs vs. any puffs for each daytime and nighttime assessment). Analyses captured the odds of reporting any daytime symptom using a combined daytime symptom score (including report of breathing problems, limitation of activity, bother, absence from school, or unscheduled doctor’s visit).[20] Models were adjusted for sex, age, education of primary caregiver, and season of sampling. Presence of pets, carpeting in the child’s bedroom, and report of smoking in the home were not associated with both exposure and outcomes and thus were not included as covariates. Analyses were performed with StataSE statistical software, version 12.0 (Stata Corp, College Station, TX). Statistical significance was defined as a p value less than 0.05 for main models and 0.1 for interaction terms.
3. Results
3.1 Participant characteristics
30 participants were enrolled in the study and contributed a total of 126 sampling days. Children on average were 9.8 years old and 33% were female, and 97% were African-American. The majority of caregivers had less than a high school education, and 100% of participants had public insurance. Daytime symptoms and albuterol use were reported on 26% and 19% of sampling days, respectively. Overnight albuterol use was reported on 18% of nights. (Table 1).
Table 1.
Participant characteristics
| N=30 participants | |
| N=126 participant days | |
| Age, mean (SD) | 9.8 (2.4) |
| Sex, n (%) female | 10 (33) |
| Race, n (%) black | 29 (97) |
| Education of primary caregiver, n (%) | |
| <8th grade | 9 (30) |
| High school | 14 (47) |
| Some college | 7 (23) |
| Public insurance, n (%) | 30 (100) |
| Any smoking by primary caregiver, n (%) | 14 (47) |
| Hours spent indoors at home, mean (SD) | |
| Total over 24 hours | 15.8 (3.8) |
| 6pm-6am | 10.4 (2.6) |
| 6am-12pm | 3.4 (2.3) |
| 12pm-6pm | 2.8 (2.1) |
| FEV1 % predicted at baseline, mean (SD) | 91.5 (14.6) |
| Any nighttime awakening, n (%) of days reported | 13 (10) |
| Any daytime symptom, n (%) of days reported | 33 (26) |
| Rescue inhaler use overnight, n (%) of days reported | 22 (18) |
| Rescue inhaler use during the day, n (%) of days reported | 28 (22) |
3.2 Housing characteristics and NO2 concentrations
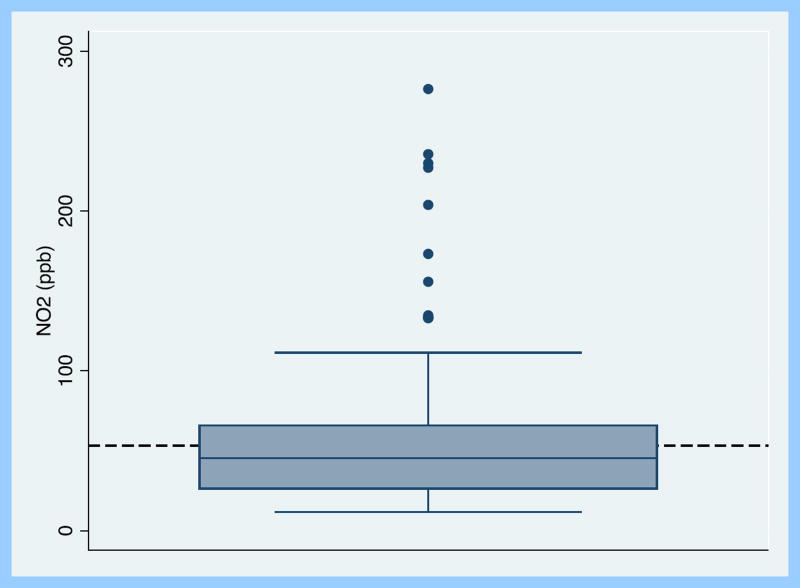
The mean(SD) indoor NO2 concentration per 24-hour sampling period was 58 (48)ppb with a median (range) of 45 (12-235)ppb (Figure 1). 47 (37%) and 15 (12%) of sampling days had 24-hour indoor concentrations above the EPA mean annual outdoor limit of 53 ppb and one-hour outdoor limit of 100 ppb, respectively. 106 (84%) of sampling days fell above the mean annual indoor guideline of 21 ppb suggested by WHO, and 13 (10%) sampling days fell above the 1-hour indoor suggested limit of 106 ppb. All homes had gas stoves and gas furnaces. Stove use at any point over the 24-hour period was reported on 72% of sampling days. 37% of the sampling days took place in the Baltimore cold season (November 1-March 31). The majority of homes were row houses, which are homes that share a wall with immediate neighbors on either side. (Table 2). There was no difference in 24-hour average NO2 concentration by housing type or distance to the curb. All homes were located within a 6-mile radius of the outdoor NO2 sampling site. There was minimal correlation between daily indoor and daily outdoor NO2 concentrations (R2=0.02, p=0.01). NO2 concentrations were higher in the cold season compared to the non-cold season (60 vs. 37 ppb, p<0.001), and indoor 24-hour NO2 concentration tended to be higher on days when windows were closed (69 vs. 52 ppb, p=0.06). Smoking status of the caregiver was not associated with 24-hour indoor NO2 concentration.
Figure 1.
Boxplot of mean 24-hour indoor nitrogen dioxide concentration across sampling period. Dashed line indicates ambient annual mean limit of 53 ppb (US EPA).
Table 2.
Housing characteristics
| n=30 participants | |
| n=126 participant days | |
| Housing type, n (%) | |
| Row House | 22 (73) |
| Apartment | 6 (20) |
| Other | 2 (7) |
| Rental home, n (%) of homes | 25 (83) |
| Gas stove, n (%) of homes | 30 (100) |
| Gas furnace, n (%) of homes | 30 (100) |
| Distance from curb to front door, n (%) | |
| <20 feet | 18 (60) |
| ≥20 feet | 12 (40) |
| Cold season, n (%) of days | 46 (37) |
| Windows open for greater than 10 minutes, n (%) of days | 79 (63%) |
| 24-hour average indoor [NO2] (ppb), mean (SD) | 58 (48) |
| 24-hour average indoor [NO2] (ppb), median (range) | 45 (12–276) |
| 24-hour average outdoor [NO2] (ppb), mean (SD) | 27 (9) |
| 24-hour average outdoor [NO2] (ppb), median (range) | 26 (5–53) |
| Frequency of daily cooking appliance use, n (%) of sampling days | |
| No use | 35 (28) |
| Reported use in one 8-hour period | 38 (31) |
| Reported use in two 8-hour periods | 32 (26) |
| Reported use in three 8-hour periods | 18 (15) |
| In subset of homes with detailed time activity information | |
| n=18 participants | |
| n=79 sampling days | |
| Duration of daily cooking appliance use, n (%) of sampling days | |
| No use | 21 (26) |
| 15–90 minutes | 38 (48) |
| >90 to 150 minutes | 13 (16) |
| >150 minutes | 8 (10) |
3.3 Relationship between cooking appliance use and 24-hour NO2 concentration
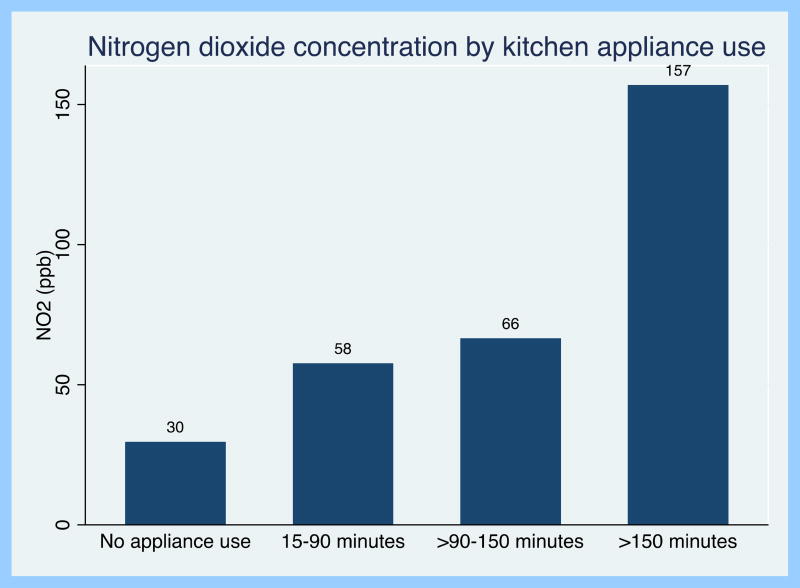
Homes had higher NO2 concentrations on days that caregivers reported any use of the cooking appliance compared to days where they reported no gas stove use (66 vs. 37 ppb; p=0.002). In the homes with detailed time activity diaries (n=18), stoves were used between 0–570 minutes/24 hour period. The longer duration of stove use was associated with higher NO2 concentration in a dose-dependent fashion. For example, on days that the stove was used for greater than 150 minutes the mean 24-hour NO2 concentration was 157ppb (NO2 concentration >150 vs ≤150 minutes p<0.001) (Figure 2). Overall, in bivariate analysis, each hour of cooking appliance use was associated with a 21ppb increase in 24-hour indoor NO2 concentration (p<0.001). When adjusting for confounders (season, outdoor NO2 concentration, and window opening), each hour of cooking appliance use was associated with an 18ppb increase in 24-hour NO2 concentration (p<0.001).
Figure 2.
24-hour indoor nitrogen dioxide concentration by kitchen appliance use
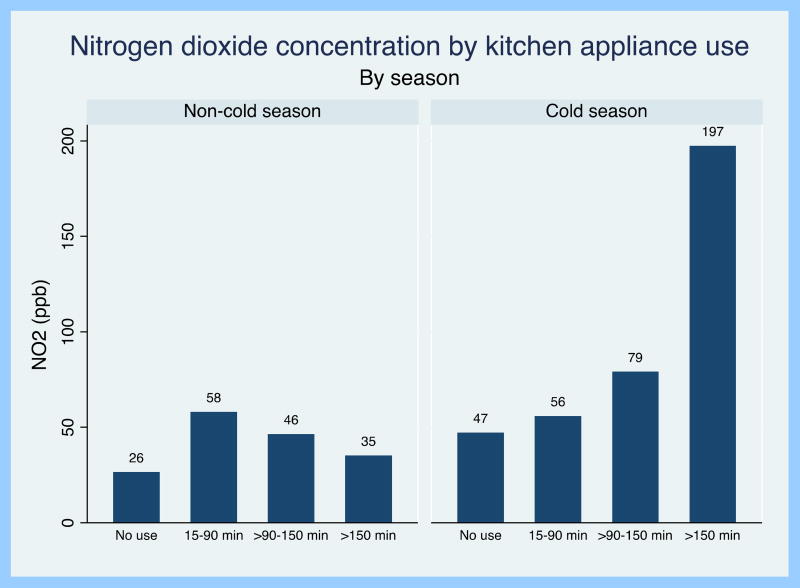
There was evidence of effect modification by both season and window opening on the relationship between duration of cooking appliance use and indoor NO2 (p-interaction <0.001 and 0.004, respectively). Mean duration of cooking time was longer in the cold vs. non-cold season (132 vs. 49 minutes, p<0.001) and duration of cooking appliance use was significantly associated with 24-hour NO2 concentration in the cold season only (Figure 3). In stratified analyses, each hour of cooking appliance use was associated with a 25 ppb increase in 24-hour NO2 concentration during the cold season (p<0.001), but there was no statistically significant association during the non-cold season (p=0.23). Similarly, the effect of cooking time on 24-hour NO2 concentration was greater on days when windows were shut (35 ppb increase in NO2 concentration per hour of cooking; p<0.001) compared to days when the windows were open for at least 10 minutes (14 pbb increase in NO2 concentration per hour of cooking; p<0.001), even when accounting for season. There was no significant interaction between duration of cooking, season, and window opening.
Figure 3.
24-hour indoor nitrogen dioxide concentration by kitchen appliance use, stratified by season
3.4 Relationship between 24-hour NO2 concentration and asthma outcomes
Higher 24-hour indoor NO2 concentration was associated with a greater odds of overnight inhaler use. Specifically, a 10-fold increase in 24-hour indoor NO2 concentration was associated with a higher odds of overnight inhaler use on the evening following the exposure (bivariate analysis OR=4.8, p=0.03; multivariate analysis OR=4.9, p=0.04). For example, 24-hour NO2 concentration measured from 4pm on Day 1 to 4pm on Day 2 was associated with a corresponding increase in overnight inhaler use from Day 2 to 3. Indoor NO2 concentrations were not associated with report of daytime albuterol use, daytime symptom score or lung function (Table 3).
Table 3.
Relationship between 24-hour indoor NO2 concentration and asthma outcomes
| Bivariate analysis | Multivariate analysis* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| OR per log increase in 24-hour |
CI | p | OR per log increase in 24-hour |
CI | P | |
|
| ||||||
| indoor NO2 | indoor NO2 | |||||
| Outcome | ||||||
| Any nighttime awakening | 0.79 | 0.09, 7.26 | 0.84 | 0.73 | 0.07, 7.68 | 0.79 |
| Any daytime symptom | 0.68 | 0.11, 4.10 | 0.67 | 0.63 | 0.08, 4.72 | 0.65 |
| Rescue inhaler use overnight | 4.81 | 1.16, 20.00 | 0.03 | 4.90 | 1.09, 22.00 | 0.04 |
| Rescue inhaler use during the day | 2.00 | 0.44, 9.02 | 0.37 | 1.96 | 0.34, 11.29 | 0.45 |
Models adjusted for gender, age, caregiver education, and season
4. Discussion
We have found that use of gas stoves is associated with an increase in 24-hour NO2 concentrations during the cold season and when windows in the home remain closed. Specifically, across the entire study, each hour of cooking appliance use was associated with an 18ppb increase in 24-hour NO2 concentration, and in the cold season, each hour of cooking appliance use increased 24-hour NO2 concentration by 25ppb. Cooking while windows are closed is associated with even higher 24-hour NO2 concentrations, with one hour of cooking associated with a 35ppb increase in 24-hour NO2 concentration. Furthermore, these higher NO2 concentrations are associated with an increase use of overnight rescue inhaler in children with asthma. The results of this study clarify the importance of daily increases in NO2 concentration on asthma health and emphasize indoor stove use as an important contributor to these elevated concentrations.
Experiments in controlled kitchen environments have shown that gas stoves can generate high short-term concentrations of NO2[22], and a modeling study conducted in the United Kingdom estimated that regular use of a gas cooking appliance could be associated with a 1-hour mean NO2 exposure of 150ppb.[23] Our real-life study confirms that that the use of the stove for 2 hours may increase 24-hour indoor NO2 concentrations to levels close to 200 ppb, a value above both the annual and 1-hour EPA ambient limits and WHO recommended indoor air guidelines. A study in Boston found that cooking on a gas stove for more than an hour a day was associated with a 5.7 ppb higher indoor NO2 concentration measured over a period of 3–4 days.[24] However, our study suggests that one hour of cooking may increase same day indoor NO2 concentrations by as much as 25 or 35 ppb, and thus looking at NO2 concentrations over several days likely underestimates the elevated NO2 exposure on the day of cooking. Indeed, prior research from our group found week-long indoor mean NO2 concentration in similar Baltimore city homes to be 30ppb.[6] Our study offers insight into how every day cooking behaviors impact daily NO2 concentrations in a real-world, urban setting. Our ability to capture detailed time activity diaries provides novel information on how duration of cooking time impacts daily NO2 concentration.
Our results support prior research showing that indoor NO2 concentrations are higher during the cold season [25, 26] . This may be partly explained by the higher cooking time during the cold seasons which has also been shown in a prior study.[27] In addition, we found that the kitchen appliance use increased 24-hour NO2 concentration by a larger amount in the cold season compared to the non-cold season. In a study of over 1,000 homes in Albuquerque, NM, 2-week average NO2 concentrations in homes with gas stoves averaged 14 ppb higher than homes with electric cooking appliances during the winter season and the difference in concentration was less pronounced in the summer[28]. Similarly, we found that an hour of stove use increases 24-hour NO2 by 25ppb in the cold season compared to 4ppb in the non-cold season; this did not appear to be driven by differences in outdoor NO2. There was a trend towards higher 24-hour NO2 concentrations on days when the windows were opened, and duration of cooking impacted 24-hour NO2 concentrations to a greater extent on days when windows were not opened, supporting prior research that home ventilation is an important predictor of indoor NO2 concentration[29]. The impact of a greater increase in NO2 concentration during the cold season is likely largely explained by lower home ventilation and residual confounding by open windows or doors.
Though studies of indoor air quality suggest that average NO2 concentrations over several days are associated with worse asthma health, little data exists that evaluates the effect of daily, hourly, or shorter-term increases in indoor NO2 concentrations on asthma morbidity in children. In a study of 174 Australian school children with asthma, daily home monitoring captured indoor NO2 concentration from the time the child came home from school until the time they went to sleep. Mean maximum daily kitchen NO2 concentration was 38 ppb, and higher short term NO2 concentration was associated with increase in nighttime asthma attacks, and a borderline statistically significant increase in daily inhaler use.[30] In a study of 125 self-reported asthmatics, a standard deviation increase (43.8ppb) in short-term residential NO2 concentration measured over an average of 4.5 hours was associated with increased report of chest tightness, dyspnea on exertion, and asthma attacks[18]. Despite limited power to study health effects given our small sample size, our findings of an increase in overnight albuterol following exposure to a higher previous-day 24 hour NO2 are also suggestive of adverse effects of short-term indoor NO2 exposure. Though there are limited indoor studies, several outdoor NO2 studies also suggest that these short exposures can impact asthma health. In a study of 138 children with asthma living in Los Angeles, mean 1-hour maximum outdoor NO2 concentration was 79.5 ppb, and higher 1-hour outdoor NO2 concentrations were associated with increased report of daily wheeze.[31] Increased 24-hour ambient NO2 concentration by an increment of 20 ppb was associated with a 9% increase in asthma symptoms and a 5% increase in use of rescue inhalers in a multi-city study of children with asthma.[32] Although there are many factors that influence asthma control, our results suggest that increases in daily indoor NO2 concentration of a magnitude similar to that in other studies can lead to meaningful worsening of symptoms, including an increase in the number of days requiring overnight rescue inhaler use.
The results of this study can also inform strategies for exposure modification. In a prior study, we found that week-long NO2 concentrations in home with gas cooking appliances decreased following replacement of the gas stove with an electric stove and with installation of a HEPA and carbon filter.[33] However, replacement of a stove may not be a feasible solution for all homes, and previous research suggests that compliance with continuous use of an air purifier is relatively low, with air purifiers being used on only about 60% of days in a randomized trial of air cleaners.[34] Thus, targeted use of air purifiers or ventilation hoods during periods of use of combustion sources that contribute to short-term increases in NO2 concentrations may be associated with higher compliance, be more cost-effective, and therefore more feasible. Prior studies have shown that replacement of unflued gas heaters was associated with a decrease in indoor NO2 concentration and subsequent improvement in respiratory symptoms in school-aged children. [9, 35–37] Further research is needed to explore whether similar interventions, including intermittent use of exhaust fans or air purifiers can modify cooking-related 24-hour NO2 concentration, and in turn can impact health outcomes.
Our study has several notable limitations. The relationship between cooking duration and daily NO2 concentrations reflects the housing environment, climate, and household behaviors in a sample of participants in urban Baltimore, where the majority of homes are rowhouses and use gas-fueled furnaces. Whether this relationship is similar in different locations and housing types, or in a population with a different socioeconomic status than the participants in this study, is unknown. We do not have information on kitchen ventilation (i.e. ventilation hoods), however previous research in a similar cohort has shown that the majority of gas stoves in Baltimore are unvented [38, 39], and we do not know if using the oven versus stove top alone differentially impacted 24-hour indoor NO2 concentration. Although we have information on whether windows were open for more than 10 minutes, we do not have detailed information on the duration of window opening, or information on location of opened windows. Furthermore, the outdoor monitoring data used in this study may not reflect the outdoor NO2 concentration immediately outside the home.
5. Conclusion
In a population of children with asthma living in Baltimore city, cooking with a gas stove for one hour was associated with a 25 ppb-increase in 24-hour indoor NO2 concentration in the cold season and a 35 ppb increase when windows were closed. Children living in homes with higher 24-hour NO2 concentrations reported increased use of asthma rescue medication in the evening following exposure. These results suggest the need for further research on feasibility and effectiveness of in-home and behavior modifications to reduce exposure to NO2 and improve health outcomes related to intermittent use of cooking-related NO2 sources in the home.
Highlights.
Nitrogen dioxide (NO2) exposure is associated with poor childhood asthma control
Indoor sources of NO2 include gas cooking stoves
Gas stove use is associated with higher indoor 24-hour NO2 concentration
Higher 24-hour NO2 concentration is associated with increased rescue inhaler use
Acknowledgments
Funding
Research reported in this manuscript was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under award numbers P01ES018176, P50ES015903, F32ES022115, and K23ES025781.
This publication was developed under Assistance Agreement No. 83451001 and 83213901 awarded by the U.S. Environmental Protection Agency. It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement on human subjects research
The Johns Hopkins Medical IRB approved the protocol and Informed consent was obtained from all individual participants included in the study. All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Centers for Disease Control and Prevention. Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001 -- 2009. MMWR Morbidity and mortality weekly report. 2011;60(17):547. [PubMed] [Google Scholar]
- 2.World Health Organization. Global status report on noncommunicable diseases 2010; World Health Organization. 2010. [Google Scholar]
- 3.National Heart, Lung, and Blood Institute, National Asthma Education and Prevential Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 [Google Scholar]
- 4.United States Environmental Protection Agency. Integrated Science Assessment for Oxides of Nitrogen. 2016 Available at https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=310879.
- 5.Belanger K, Gent JF, Triche EW, Bracken MB, Leaderer BP. Association of indoor nitrogen dioxide exposure with respiratory symptoms in children with asthma. Am J Respir Crit Care. 2006;173(3):297. doi: 10.1164/rccm.200408-1123OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansel NN, Breysse PN, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, Moore JL, Cuhran JL, Diette GB. A longitudinal study of indoor nitrogen dioxide levels and respiratory symptoms in inner-city children with asthma. Environ Health Perspect. 2008;116(10):1428. doi: 10.1289/ehp.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrett MH, Hooper MA, Hooper BM, Abramson MJ. Respiratory symptoms in children and indoor exposure to nitrogen dioxide and gas stoves. Am J Respir Crit Care. 1998;158(3):891. doi: 10.1164/ajrccm.158.3.9701084. [DOI] [PubMed] [Google Scholar]
- 8.Hasselblad V, Eddy DM, Kotchmar DJ. Synthesis of environmental evidence: nitrogen dioxide epidemiology studies. J Air Waste Manage Assoc. 1992;42(5):662–671. doi: 10.1080/10473289.1992.10467018. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie-Bennett J, Pierse N, Wickens K, Crane J, Howden-Chapman P. The respiratory health effects of nitrogen dioxide in children with asthma. European Respiratory Journal. 2011;38(2):303–309. doi: 10.1183/09031936.00115409. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. World Health Organization. 2006. UNAIDS. Air quality guidelines: global update 2005. [Google Scholar]
- 11.Levy J. Impact of residential nitrogen dioxide exposure on personal exposure: an international study. J Air Waste Manage Assoc. 1998;48(6):553–560. doi: 10.1080/10473289.1998.10463704. [DOI] [PubMed] [Google Scholar]
- 12.Neas LM, Dockery DW, Ware JH, Spengler JD, Speizer FE, Ferris BG. Association of indoor nitrogen dioxide with respiratory symptoms and pulmonary function in children. Am J Epidemiol. 1991;134(2):204–219. doi: 10.1093/oxfordjournals.aje.a116073. [DOI] [PubMed] [Google Scholar]
- 13.Kattan M, Gergen PJ, Eggleston P, Visness CM, Mitchell HE. Health effects of indoor nitrogen dioxide and passive smoking on urban asthmatic children. J Allergy Clin Immun. 2007;120(3):618–624. doi: 10.1016/j.jaci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Energy Information Administration (EIA) Residential Energy Consumption Survery (RECS) 2015 [Google Scholar]
- 15.Brauer M, Ryan PB, Suh HH, Koutrakis P, Spengler JD, Leslie NP, Billick IH. Measurements of nitrous acid inside two research houses. Environ Sci Technol. 1990;24(10):1521–1527. [Google Scholar]
- 16.Franklin P, Runnion T, Farrar D, Dingle P. Comparison of peak and average nitrogen dioxide concentrations inside homes. Atmos Environ. 2006;40(38):7449–7454. [Google Scholar]
- 17.Nitschke M. Respiratory health effects of nitrogen dioxide exposure and current guidelines. Int J Environ Res Public Health. 1999;9(1):39–53. [Google Scholar]
- 18.Smith B, Nitschke M, Pilotto L, Ruffin R, Pisaniello D, Willson K. Health effects of daily indoor nitrogen dioxide exposure in people with asthma. ERJ. 2000;16(5):879–885. doi: 10.1183/09031936.00.16587900. [DOI] [PubMed] [Google Scholar]
- 19.US Environmental Protection Agency Air Quality System Database. 2015 Available at: https://www3.epa.gov/ttn/airs/airsaqs/
- 20.Santanello NC, Davies G, Galant SP, Pedinoff A, Sveum R, Seltzer J, Seidenberg BC, Knorr BA. Validation of an asthma symptom diary for interventional studies. Arch Dis Child. 1999;80(5):414–420. doi: 10.1136/adc.80.5.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data second Edition. Oxford statistical science series. 2002;1(25) ALL-ALL. [Google Scholar]
- 22.Dennekamp M, Howarth S, Dick C, Cherrie J, Donaldson K, Seaton A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup Environ Med. 2001;58(8):511–516. doi: 10.1136/oem.58.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dimitroulopoulou C, Ashmore M, Byrne M, Kinnersley R. Modelling of indoor exposure to nitrogen dioxide in the UK. Atmos Environ. 2001;35(2):269–279. [Google Scholar]
- 24.Baxter LK, Clougherty JE, Laden F, Levy JI. Predictors of concentrations of nitrogen dioxide, fine particulate matter, and particle constituents inside of lower socioeconomic status urban homes. J Expo Sci Environ Epidemiol. 2007;17(5):433–444. doi: 10.1038/sj.jes.7500532. [DOI] [PubMed] [Google Scholar]
- 25.Garrett MH, Hooper MA, Hooper BM. Nitrogen dioxide in Australian homes: levels and sources. J Air Waste Manage Assoc. 1999;49(1):76–81. doi: 10.1080/10473289.1999.10463781. [DOI] [PubMed] [Google Scholar]
- 26.Zota A, Adamkiewicz G, Levy JI, Spengler JD. Ventilation in public housing: implications for indoor nitrogen dioxide concentrations. Indoor Air. 2005;15(6):393–401. doi: 10.1111/j.1600-0668.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 27.Schwab M, McDermott A, Spengler JD, Samet JM, Lambert WE. Seasonal and yearly patterns of indoor nitrogen dioxide levels: data from Albuquerque, New Mexico. Indoor Air. 1994;4(1):8–22. [Google Scholar]
- 28.Lambert W, Samet J, Hunt W, Skipper B, Schwab M, Spengler J. Nitrogen dioxide and respiratory illness in children. Part II: Assessment of exposure to nitrogen dioxide. Res Rep Health Eff Inst. 1993;58:33–50. discussion 51-80. [PubMed] [Google Scholar]
- 29.Baxter LK, Clougherty JE, Paciorek CJ, Wright RJ, Levy JI. Predicting residential indoor concentrations of nitrogen dioxide, fine particulate matter, and elemental carbon using questionnaire and geographic information system based data. Atmos Environ. 2007;41(31):6561–6571. doi: 10.1016/j.atmosenv.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nitschke M, Pilotto LS, Attewell RG, Smith BJ, Pisaniello D, Martin J, Ruffin RE, Hiller JE. A cohort study of indoor nitrogen dioxide and house dust mite exposure in asthmatic children. J Occup Env Med. 2006;48(5):462–469. doi: 10.1097/01.jom.0000215802.43229.62. [DOI] [PubMed] [Google Scholar]
- 31.Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001;12(2):200–208. doi: 10.1097/00001648-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006;164(6):505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 33.Paulin LM, Diette GB, Scott M, McCormack MC, Matsui EC, Curtin-Brosnan J, Williams DL, Kidd-Taylor A, Shea M, Breysse PN, Hansel NN. Home interventions are effective at decreasing indoor nitrogen dioxide concentrations. Indoor Air. 2014;24(4):416–424. doi: 10.1111/ina.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, Williams D, Yuan J, Bernert JT, Rand C. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165(8):741–748. doi: 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks GB, Ezz W, Aust N, Toelle BG, Xuan W, Belousova E, Cosgrove C, Jalaludin B, Smith WT. Respiratory health effects of exposure to low-NOx unflued gas heaters in the classroom: a double-blind, cluster-randomized, crossover study. Environ Health Perspect. 2010;118(10):1476. doi: 10.1289/ehp.1002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilotto LS, Nitschke M, Smith BJ, Pisaniello D, Ruffin RE, McElroy HJ, Martin J, Hiller JE. Randomized controlled trial of unflued gas heater replacement on respiratory health of asthmatic schoolchildren. Int J Epidemiol. 2004;33(1):208. doi: 10.1093/ije/dyh018. [DOI] [PubMed] [Google Scholar]
- 37.Howden-Chapman P, Pierse N, Nicholls S, Gillespie-Bennett J, Viggers H, Cunningham M, Phipps R, Boulic M, Fjallstrom P, Free S, Chapman R, Lloyd B, Wickens K, Shields D, Baker M, Cunningham C, Woodward A, Bullen C, Crane J. Effects of improved home heating on asthma in community dwelling children: randomised controlled trial. BMJ. 2008;337:a1411. doi: 10.1136/bmj.a1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diette GB, Hansel NN, Buckley TJ, Curtin-Brosnan J, Eggleston PA, Matsui EC, McCormack MC, Williams DL, Breysse PN. Home indoor pollutant exposures among inner-city children with and without asthma. Environ Health Perspect. 2007;115(11):1665. doi: 10.1289/ehp.10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breysse PN, Buckley TJ, Williams D, Beck CM, Jo SJ, Merriman B, Kanchanaraksa S, Swartz LJ, Callahan KA, Butz AM, Rand CS, Diette GB, Krishnan JA, Moseley AM, Curtin-Brosnan J, Durkin NB, Eggleston PA. Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore. Environ Res. 2005;98(2):167–176. doi: 10.1016/j.envres.2004.07.018. [DOI] [PubMed] [Google Scholar]