Figure 3. The MYS‐1 HAT complex is recruited to the daf‐16 promoter region and contributes to histone acetylation.
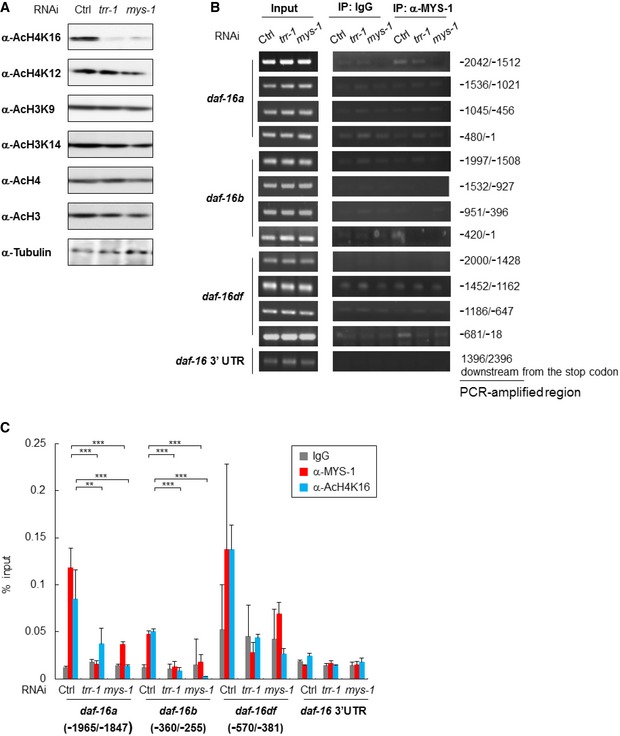
- Whole‐worm lysates isolated from the indicated RNAi‐treated N2 worms at day 2 adulthood were subjected to immunoblot analysis using the indicated antibodies. Representative images of three independent experiments are shown.
- MYS‐1 binding was examined by ChIP–PCR using crosslinked DNA–protein complexes isolated from the indicated RNAi‐treated N2 worms at day 2 adulthood with anti‐Tip60/MYS‐1 and control IgG antibody. PCR amplification was done with specific primers for the daf‐16 promoter region and the daf‐16 3′UTR. Representative images of two independent experiments are shown. Genomic DNA in the input samples was used as a positive control.
- MYS‐1 binding and histone H4K16 acetylation status were examined by ChIP–qPCR using crosslinked DNA–protein complexes isolated from the indicated RNAi‐treated N2 worms at day 2 adulthood with anti‐Tip60/MYS‐1, anti‐AcH4K16 and control IgG antibody. The bars represent the percentage of total input DNA for each ChIP sample, and error bars represent the SD derived from three independent experiments. **P < 0.01, ***P < 0.001, one‐way ANOVA followed by Tukey's test. See also Fig EV3.
Source data are available online for this figure.
