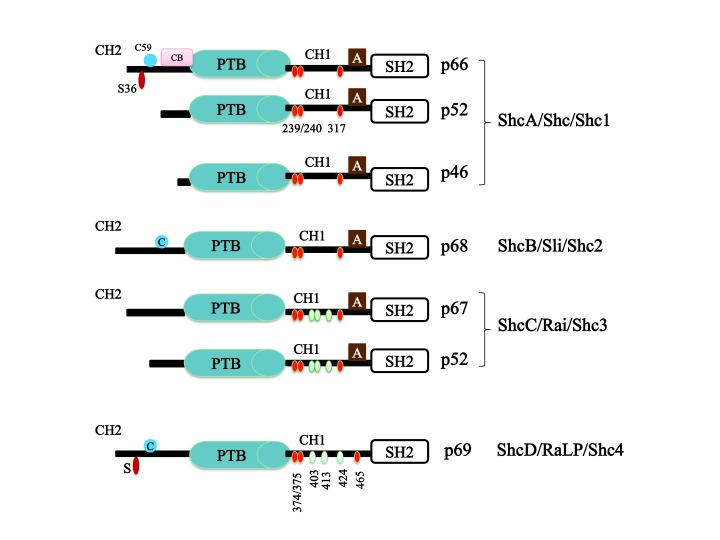
Figure 1.
Schematic representation of the structural modularity of the Shc family of proteins. Shc proteins share the same structural hallmarks of highly conserved PTB and SH2 domains and poorly conserved CH2 and CH1 domains. Three conserved tyrosine phosphorylation sites exist in the CH1 domain (yellow). In ShcC and ShcD/RaLP, additional tyrosine phosphorylation residues (non-conserved) are present in the same CH1 domain (green). SER36 in the p66ShcA-CH2 domain is responsible for the oxidative-stress response function. RaLP-CH2 also contains a putative serine site for phosphorylation. A cysteine (C) residue in the p66ShcA-CH2 domain of ShcB and ShcD/RaLP (blue) is involved in oligomerization. A cytochrome c (CB) binding site is present only in p66ShcA but not in other Shc proteins (pink). An Adaptin binding motif (A) is present in all Shc proteins except ShcD/RaLP (cream-coloured) (adapted from Melanie and Jones, 2012).