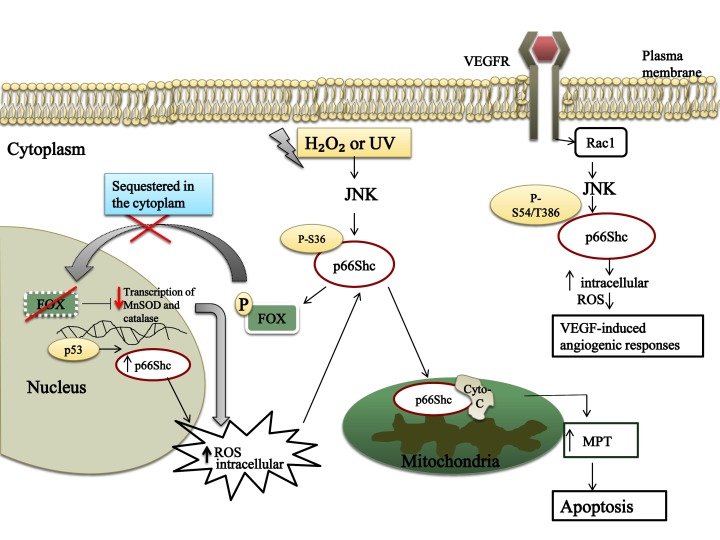
Figure 3.
p66ShcA has a key role in the oxidative stress response. p66ShcA is phosphorylated on Ser36 via the activation of stress kinases such as JNK after the exposure of cells to oxidative stress agents (e.g., H2O2 and UV). This results in translocation of a fraction of p66ShcA into the mitochondrial intermembrane space, where it binds physically with cytochrome c (Cyto-c), which results in a mitochondrial permeability transition (MPT). The release of cytochrome c results in apoptosome formation, initiating the apoptotic process. p66ShcA mediates the transcription factor FOX phosphorylation, which results in an inhibition in the transcription of scavenging enzymes such as catalase and MnSOD. Upon VEGF stimulation in endothelial cells, p66ShcA is phosphorylated at Ser54 and Thr286 in response to Rac1 activation. Consequentially, the produced intracellular reactive oxygen species (ROS) initiate the VEGF-mediated angiogenic response.