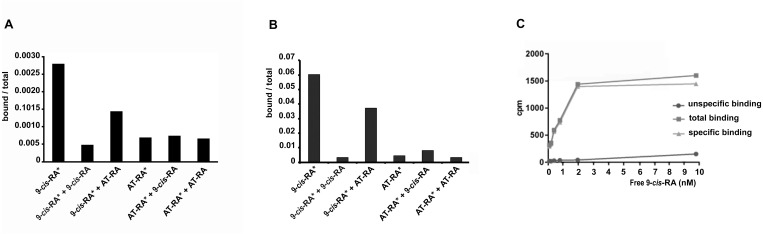
Figure 2. Binding of retinoic acids to TaRXR.
(A) Single point analysis of binding preference of T. adhaerens RXR (thrombin cleaved) to 3H-labelled 9-cis-RA over all-trans-RA. Radioactive 9-cis-RA (9-cis-RA∗) binds at a concentration of 4 nM to 200 nanograms of T. adhaerens RXR. 200-fold excess of unlabeled 9-cis-RA displaces more than 80% of labeled 9-cis-RA from binding to T. adhaerens RXR (9-cis-RA∗ + 9-cis-RA) while the same molar excess of all-trans-RA (9-cis-RA∗ + AT-RA) which is likely to contain approximately 1% spontaneously isomerized 9-cis- RA, competes away less than 50 % of bound 3H-labeled 9-cis-RA. Radioactive 3H-labeld all-trans-RA (AT-RA∗) at identical conditions binds only slightly more than the observed non-specific binding. This interaction is not displaced by the excess of non-labeled 9-cis-RA (AT-RA∗ + 9-cis-RA) nor non-labeled all-trans-RA (AT-RA∗ + AT-RA). Results are expressed as a ratio of the radioactivity bound to TaRXR/total radioactivity used for the binding at the given condition. (B) Analysis of binding properties of T. adhaerens RXR (in the form of GST-TaRXR) to 3H-labelled 9-cis-RA and 3H-labelled all-trans-RA. The experiment differs from the experiment shown in A in 5-fold greater amount of radioactive all-trans-RA (and therefore only 40-fold excess of non-radioactive competitors). The experiment shows identical binding properties of GST-TaRXR as those observed with thrombin cleaved TaRXR. (C) Kinetic analysis of binding of 3H-labeled 9-cis-RA to T. adhaerens RXR prepared as GST-fusion protein (GST-TaRXR). The plateau is reached at around 3 to 5 × 10−9 M.