Abstract
Increased sugar consumption has been proposed to be a risk factor for obesity-related metabolic disorders. The objective of this study was to investigate the anti-inflammatory effect of turanose in Raw 264.7 macrophages. Turanose (3-O-α-D-glucosyl-D-fructose), an isomer of sucrose, naturally exists in honey. For these studies, macrophages were treated with total glucose (Glu), 50% Glu/50% turanose (T50), 25% Glu/75% turanose (T75), and 100% turanose (T100), each with a total concentration of 25 mM in cell media. Expressions of inflammatory enzymes and cytokines were analyzed. Cell viability was not affected in the turanose treated groups compared to the Glu group. Lipopolysaccharide and glucose-induced nitric oxide production, protein expression of inducible nitric oxide synthase, COX-2, and superoxide dismutase 2, and mRNA expression levels of interleukin (IL)-1β and IL-18 were significantly suppressed by turanose treatment. These results demonstrate that turanose exerts anti-inflammatory effects in vitro, and possesses potential to serve therapeutic functional sweetener for testing in vivo and in clinical trials.
Keywords: Turanose, Sweetening agents, Macrophages, Inflammation
INTRODUCTION
Sucrose is a disaccharide that is composed of glucose and fructose. Sucrose is used for a variety of applications, especially in the food industry where it is used as a sweetener and as a standard for sweetness. However, excessive intake of sucrose can increase an individual’s susceptibility to chronic diseases, including tooth decay, obesity, and type 2 diabetes.1 Sucrose is rapidly hydrolyzed into glucose and fructose in human intestines and increases the blood glucose level. A well-controlled blood glucose level is thought to be beneficial for treating excessive inflammatory responses and related metabolic diseases.
Inflammation is related to the pathogenesis of many diseases, including cardiovascular disease, arthritis, and atherosclerosis.2,3 Inflammation is initiated by bacterial infections and extrinsic toxic chemicals, and results in the injury and death of normal cells. Tissue injury induced by these factors leads to the release of inflammatory mediators, including interleukin (IL)-18, IL-1β, and nitric oxide (NO), from macrophages, monocytes, and leukocytes.4 Macrophages recognize pathogenic substances via cell surface receptors and initiate inflammatory responses by secreting various pro-inflammatory mediators.5 Lipopolysaccharide (LPS) is a well characterized stimulant for macrophage and induces the production of inflammatory mediators, such as NO and immune cytokines, including IL-1β and IL-18.6 NO is synthesized by nitric oxide synthase (NOS), an enzyme which has roles in various and diverse physiological processes. An inducible nitric oxide synthase (iNOS) is expressed in Raw 264.7 cells exposed to specific stimulants, including cytokines and bacterial LPS. Once stimulated, iNOS produces significantly large amounts of NO that mediates acute and chronic inflammation.7
COX-2 is induced by cytokines and extracellular stimuli in activated cells. During inflammation, COX-2 expression is induced in many cell types, including monoctyes, endothelial cells, and macrophages.8 Several studies have shown that overexpression of iNOS or COX-2 may contribute to disease-related inflammation.9,10 A group of oxidoreductases, including superoxide dismutase (SOD) which catalyzes the dismutation of O2− into oxygen and H2O2, plays a major role in cellular defenses against O2− and peroxynitrite.11 IL-1β and IL-18 are pro-inflammatory cytokines which are closely related and have similar three-dimensional structure. Furthermore, excessive production of IL-1β and IL-18 is known to cause systemic inflammatory diseases.12
Excessive consumption of sugar is a public concern and is one of the risk factors that contribute to the development of obesity, diabetes, and related chronic disease.13 Recently, new low-calorie added sugars have been developed to manage these metabolic diseases. Turanose (3-O-α-D-glucosyl-D-fructose) is a sucrose isomer that naturally exists in honey and provides one-half of the sweetness provided by sucrose.14 Turanose is a byproduct of the synthetic reaction of linear α-(1,4)-glucan from sucrose, and this reaction can be catalyzed by a recombinant form of an amylosucrase that was originally identified in Neisseria polysaccharea.15 The hydrolysis rate of turanose has been reported to result in 54% sucrose and 6% maltose at a 5 mM concentration in a rat intestinal enzyme mixture.16 Turanose is more slowly hydrolyzed to glucose and fructose in the intestine compared with sucrose, and this represents the potential for turanose to serve as a low glycemic alternative sweetener.17 Support for turanose as a sweetener substitute for sucrose and for the control adipogenesis have also been demonstrated in vitro.18
We hypothesize that turanose as a representative alternative sweetener can substitute for sucrose and mediates beneficial effects for the management of chronic metabolic diseases. However, few studies have investigated the functional roles of turanose in inflammation. Therefore, the objective of this study was to demonstrate the anti-inflammatory effects of turanose in murine macrophage cells and the potential of this sucrose isomer to serve as functional alternative sweetener for sugar.
MATERIALS AND METHODS
1. Materials
Turanose (3-O-α-D-glucosyl-D-fructose; CAS No. 547-25-1) was purchased from Carbosynth Limited (Berkshire, UK) (Fig. 1). FBS, penicillin, and streptomycin were purchased from Invitrogen (Carlsbad, CA, USA). Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from WelGene (Daegu, Korea), MTT, LPS, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Figure 1.
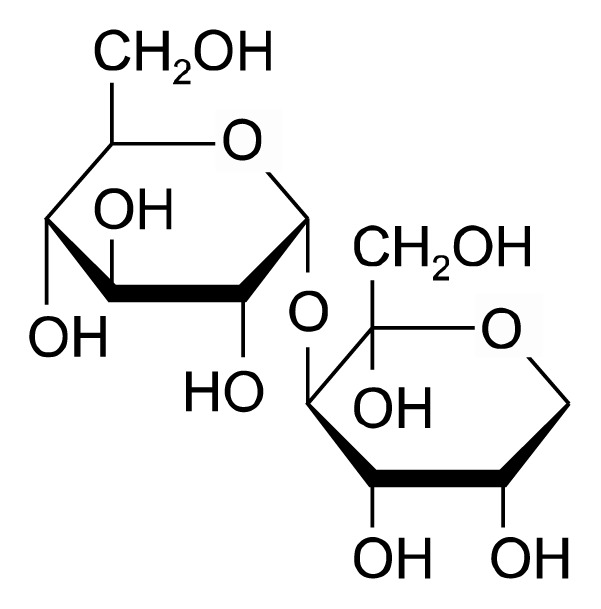
The chemical structure of turanose.
2. Cell Culture
The mouse macrophage cell line, Raw 264.7 (American Type Culture Collection, Rockville, MD, USA), was cultured in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin (100 U/mL and 100 μg/mL). Cells were maintained in 5% CO2 at 37°C.
Raw 264.7 cells were seeded in 6-well culture plates. After 24 hours, the cells were activated with 50 ng/mL LPS (Escherichia coli O111:B4) and treated with a total 25 mM concentration of glucose and turanose in various proportions in cell media for 24 hours. These proportion included: 100% glucose (Glu), 50% Glu/50% turanose (T50), 25% Glu/75% turanose (T75), and 100% turanose (T100).
3. Cell viability assay
Cell viability was assessed by using a colorimetric MTT assay.19 Briefly, Raw 264.7 macrophages were seeded in 96-well plates and treated with various proportion of glucose (Glu) and turanose (T50, T75, and T100) as indicated above. After 24 hours, the cells were washed with PBS and incubated with a 0.5 mg/mL MTT stock solution. After 4 hours, the supernatants were aspirated and DMSO was added to dissolve the formazan crystals present in each well. Absorbance values were then measured with a microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 560 nm. The results are expressed as a percentage of the absorbance value for the control group which was set to 100%.
4. Nitric oxide production assay
The LPS-stimulated inflammatory response in the Raw 264.7 cells was assessed by measuring NO production present in cell culture media as previously described.10 Briefly, cells were seeded in 96-well plates and activated by 50 ng/mL LPS. After 24 hours, NO levels were measured in the cell culture media supernatants with Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylethylenediamine dihydrochloride, and 5% H3PO4 solution). The media supernatants were transferred to new 96-well plates and mixed with Greiss reagent. After an incubation in the dark for 15 minutes, the absorbance values at 540 nm were measured with a microplate reader (Molecular Devices). The results are expressed as the percentage of absorbance values relative to the control group which was set to 100% NO production.
5. Quantitative real-time PCR analysis
Total RNA was isolated from Raw 264.7 cells using TRIzol reagent (Invitrogen), and cDNA was prepared by reverse transcription with a RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania). To analyze mRNA levels, quantitative PCR was performed for each gene by using a Rotor-Gene SYBR Green PCR kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Real-time PCR was performed by using a Rotor-Gene Q instrument (Qiagen, Austin, TX, USA) that was programmed as follows: 95°C for 15 minutes, followed by 40 cycles of 94°C for 15 seconds, 55°C for 30 seconds, and 70°C for 30 seconds. All data were normalized to the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH ), which was detected as an internal control. The primers used for PCR included the followings: mouse IL-1β, 5′-ATGGCAACTGTTCCTGAACTCAACT-′3 (forward) and 5′-CAGGACAGGTATAGATTCTTTCCTTT-′3 (reverse); mouse IL-18, 5′-TTTCTGGACTCCTGCCTGC-′3 (forward) and 5′-ATTTGGAAGGTTTGAGGCGG-′3 (reverse); mouse GAPDH, 5′-AACTTTGGCATTGTGGAAGG-′3 (forward) and 5′-TGTGAGGGAGATGCTCAGTG-′3 (reverse).
6. Western blot analysis
Western blot analysis was performed as described previously.10 Briefly, Raw 264.7 cells were lysed with RIPA buffer. The concentration of total protein in each sample was determined in Bradford protein assays (Bio-Rad, Hercules, CA, USA). The cell lysates were separated with SDS-PAGE and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Membranes were blocked with a 5% non-fat dried milk solution for 1 hour. Membranes were subsequently incubated at 4°C overnight with primary antibodies, including iNOS (Santa Cruz Biotechnology, Dallas, TX, USA), COX-2 (Cell Signaling, Danvers, MA, USA), or SOD2 (Santa Cruz Biotechnology). Detection of α-tubulin (Sigma-Aldrich) was used as an internal loading control.
7. Statistical analysis
Data are presented as the mean ± SEM. All data were analyzed by using GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA). Data were analyzed with ANOVA followed by the post-hoc Tukey’s honestly significant difference (HSD) test for multiple comparisons. Statistical differences with a P-value less than 0.05 were considered significant.
RESULTS
1. Effect of turanose on cell viability
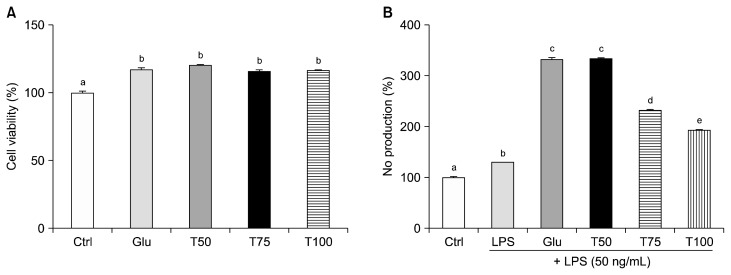
Raw 264.7 macrophages were treated with Glu, T50, T75, and T100 for 24 hours before being subjected to MTT assay (Fig. 2A). Cell viability was significantly increased in all of the treated groups compared to the non-treated control group. There was no significant differences in viability between the Glu group and any of the turanose treated groups.
Figure 2.
Effect of turanose on cell survival and nitric oxid (NO) production. (A) Cell viability of Raw 264.7 cells was analyzed using MTT assays after a 24 hours incubation with varying proportion of glucose with turanose. (B) Relative NO production levels in the cell culture media treated with 50 ng/mL lipopolysaccharide (LPS) and turanose for 24 hours were examined using Griess reagent. For a given column, data not sharing a common superscript letter significantly differ (P < 0.05). Ctrl, no glucose; Glu, 25 mM glucose; T50, T75, and T100, replaced glucose with the indicated proportion of turanose (e.g., 50%, 75%, or 100%, respectively).
2. Effect of turanose on production of nitric oxide
In the presence of 25 mM glucose, up to a 3.4-fold increase in NO production was observed (P < 0.001). In contrast, treatment with turanose significantly suppressed NO production by 30% and 42% (T75 and T100, respectively) (all P < 0.001) (Fig. 2B).
3. Effect of turanose on inflammation and related oxidative stress
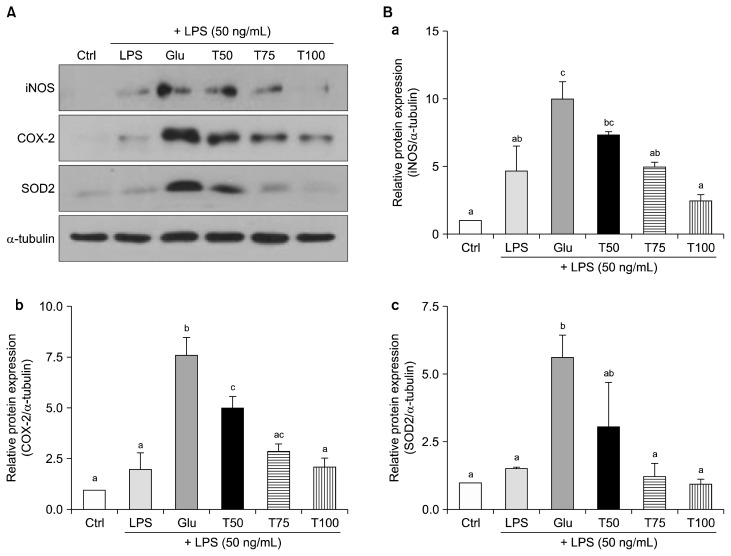
Expression levels of the pro-inflammatory enzymes, iNOS and COX-2, were analyzed to investigate the roles that turanose may have in mediating inflammation (Fig. 3). The levels of both enzymes were significantly up-regulated in the Glu group compared to the untreated control group. Conversely, both enzyme levels were down-regulated by turanose treatment compared to the Glu group (Fig. 3A, 3Ba, and 3Bb). In particular, the levels of iNOS expression significantly decreased by 51% and 75% in the T75 and T100 groups, respectively, compared to the Glu group (P < 0.05 and P < 0.001, respectively). COX-2 protein expression was down-regulated in the T50, T75, and T100 groups by 35%, 62%, and 75% (P < 0.05, P < 0.001, and P < 0.001, respectively) compared to the Glu Group. In addition, expression of the antioxidant enzyme, SOD2, increased in the Glu group by approximately 6-fold compared to the control group. The level of SOD2 was down-regulated in the T75 and T100 groups by 78% and 84%, respectively, compared to the Glu group (P < 0.05 and P < 0.01, respectively). These expression levels of SOD2 in the T75 and T100 groups were found to recover to control levels (Fig. 3Bc).
Figure 3.
Effect of turanose on inflammatory mediators, including inducible nitric oxide synthase (iNOS), COX-2, and oxidative stress-related antioxidant enzyme, superoxide dismutase 2 (SOD2). Western blot analysis was performed 24 hours after Raw 264.7 cells were incubated with lipopolysaccharide (LPS) and various proportions of turanose. (A) Representative blots and (B) quantification of band intensities (a, iNOS; b, COX-2; c, SOD2) with densitometry are shown. The expression levels of α-tubulin were used as a loading control. For a given column, data not sharing a common superscript letter significantly differ (P < 0.05). Ctrl, no glucose; Glu, 25 mM glucose; T50, T75, and T100, replaced glucose with the indicated proportion of turanose (e.g., 50%, 75%, or 100%, respectively).
4. Effect of turanose on mRNA level of interleukin-1β and interleukin-18
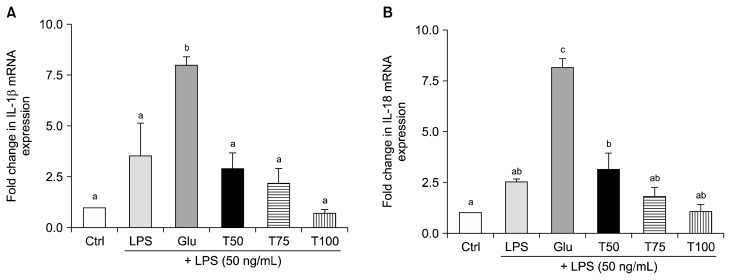
Following induction of an inflammatory response in Raw 264.7 cells treated with LPS, quantitative real-time-PCR was performed to detect mRNA levels of IL-1β and IL-18 (Fig. 4). Both genes were up-regulated by approximately 7-fold in the Glu group compared to the control group. In contrast, the mRNA levels of IL-1β were decreased by 63%, 73%, and 91% in the T50, T75, and T100 groups, respectively, compared to the Glu group (P < 0.01, P < 0.01, and P < 0.001, respectively) (Fig. 4A). The mRNA expression levels of IL-18 were down-regulated by 61%, 78%, and 87% in the T50, T75, and T100 groups, respectively, compared to the Glu group (all P < 0.001) (Fig. 4B). Moreover, in the T100 group, the mRNA levels of IL-1β and IL-18 nearly recovered to each control level.
Figure 4.
Effect of turanose on mRNA expression levels of interleukin (IL)-1β and IL-18. Expression levels of (A) IL-1β and (B) IL-18 mRNA were detected by quantitative real-time-PCR in Raw 264.7 cells treated with lipopolysaccharide (LPS) and turanose for 24 hours. Level of GAPDH was used as a loading control. For a given column, data not sharing a common superscript letter significantly differ (P < 0.05). Ctrl, no glucose; Glu, 25 mM glucose; T50, T75, and T100, replaced glucose with the indicated proportion of turanose (e.g., 50%, 75%, or 100%, respectively).
DISCUSSION
The present study demonstrates that turanose is able to suppress LPS- and glucose-induced inflammatory responses in Raw 264.7 macrophages. Specifically, NO production, expression of pro-inflammatory mediators, including iNOS and COX-2, and expression of the related antioxidant enzyme, SOD2, were significantly decreased in the presence of turanose. In addition, mRNA levels of the immune cytokines, IL-1β and IL-18, were suppressed by turanose.
LPS has previously been applied to Raw 264.7 cells at a concentration of 1 μg/mL.10 Huang et al.20 activated Raw 264.7 cells with relatively low concentration of LPS (200 ng/mL) and a dramatic increase in NO production was observed. In the present study, the treatment of Raw 264.7 cells with 50 ng/mL LPS for 24 hours significantly increased NO production in the Glu group but decreased in the T75 and T100 groups. These results demonstrate that substitution of glucose for turanose can potentially inhibit the excessive inflammatory response that is induced by both LPS and glucose.
When Raw 264.7 cells were treated with turanose, relative protein levels of iNOS, COX-2, and SOD2 were down-regulated. It has been demonstrated that the iNOS and COX-2 represent pro-inflammatory mediators and play critical roles in regulating inflammation.9,10 Moreover, SOD2 has been shown to protect cells from oxidative damage by inhibiting the activities of superoxide free radicals (O2−).11 The observed down-regulation of SOD2 expression in the present study indicates that lower level of O2− was achieved, and this is potentially a beneficial effect against inflammatory diseases. Peroxynitrite (ONOO−) is produced from the reaction between O2− and NO. Due to the strong reactive property of ONOO−, this substance causes oxidative damage to various biomolecules. Consequently, an increase in NO production has the potential to increase the amount of oxidative damage mediated by ONOO−, while an increase in SOD2 expression could enhance elimination of O2− from the body. Thus, in the present study, the observed decrease in SOD2 expression following treatment with turanose might be interpreted as a reduction in the inflammatory response, which would be consistent with the observed decrease in NO production.
It is possible that anti-inflammatory mechanisms are specifically related to IL-1β and IL-18. For example, activated IL-1β plays a key role in acute inflammation by respiratory infections and the clearing pathogen, while prolonged activation of IL-1β can leads to chronic systemic inflammation.12 IL-18 is a converted biologically active form from its inactive precursor form (pro-IL-18).21 However, to date, activation of IL-18 has only been observed in dendritic cells and macrophages.6 In the present study, turanose treatment specifically down-regulated mRNA levels of IL-1β and IL-18, which appears to indicate the anti-inflammatory effects of this sugar substitute. Recently, it was reported that stevioside, a diterpene glycoside isolated from Stevia rebaudiana, exhibited an anti-inflammatory effect by suppressing the secretion of pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6.22 In addition, supplementation with xylobiose, a dimer of xylose, down-regulated mRNA expression of IL-1β, TNF-α, and MCP-1 in db/db mice.23
As a significant and efficient energy source, sucrose is the most commonly used sweetener and rapidly raises blood glucose after intake. However, excessive consumption of sucrose is associated with metabolic diseases, such as obesity and type 2 diabetes. Therefore, various functional alternative sugar substitutes, including D-xylose, D-tagatose, and isomaltulose, have been developed and investigated for their potential to replace sucrose.24–27 For example, a disaccharide made from sucrose, isomaltulose is a well-known sugar replacement which exhibits low-glycemic properties by raising blood glucose and insulin levels at a slower rate than sucrose following its oral administration.27 A well-controlled blood glucose level is considered to be beneficial for the treatment of an excessive inflammatory response. In addition, an immune-modulating diet containing isomaltulose was shown to suppress a systemic inflammation response following gut ischemia-reperfusion injury in mice.28 Taken together, these anti-inflammatory effects of isomaltulose are consistent with the results observed in the present study for the sucrose isomer, turanose.
Only few studies have reported the hydrolysis rate of turanose using microorganisms, and these studies were limited to in vitro. Hydrolysis of turanose by rat intestinal enzymes was also reported, with a 54% rate achieved for sucrose at a 5 mM concentration level.29 However, in vivo studies are required to more fully characterize the absorption rate and metabolism of turanose. The absorption and metabolism of another sucrose isomer, isomaltulose, have been characterized with carbohydrate load tests and [14C] isomaltulose-radioisotope labeling tests in rats and humans, respectively. No significant differences were observed between the isomaltulose-treated group and the sucrose-treated group.30 Further studies to investigate the clinical implications of turanose administration are required to explore the potential applications of this molecule as a sugar substitute for humans.
The treatment of Raw 264.7 macrophages with turanose resulted in anti-inflammatory effects regulating NO production, inflammatory mediators, and cytokines. Further studies regarding the signaling pathways that mediate these anti-inflammatory effects are needed. These results demonstrate the potential of this sucrose isomer as a dietary supplement for the prevention of metabolic diseases.
ACKNOWLEDGMENTS
This research was supported by National Research Foundation of Korea (NRF-2015R1A2A1A15056119).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- 1.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–43. 10.1161/hc0902.104353 [DOI] [PubMed] [Google Scholar]
- 3.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001;358: 903–11. 10.1016/S0140-6736(01)06075-5 [DOI] [PubMed] [Google Scholar]
- 4.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol 2003;171:1473–83. 10.4049/jimmunol.171.3.1473 [DOI] [PubMed] [Google Scholar]
- 5.Hunter M, Wang Y, Eubank T, Baran C, Nana-Sinkam P, Marsh C. Survival of monocytes and macrophages and their role in health and disease. Front Biosci (Landmark Ed) 2009;14:4079–102. 10.2741/3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem 2001;276:3820–6. 10.1074/jbc.M006814200 [DOI] [PubMed] [Google Scholar]
- 7.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991;43:109–42. [PubMed] [Google Scholar]
- 8.Hla T, Ristimäki A, Appleby S, Barriocanal JG. Cyclooxygenase gene expression in inflammation and angiogenesis. Ann N Y Acad Sci 1993;696:197–204. 10.1111/j.1749-6632.1993.tb17152.x [DOI] [PubMed] [Google Scholar]
- 9.Zong Y, Sun L, Liu B, Deng YS, Zhan D, Chen YL, et al. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS One 2012;7:e44107. 10.1371/journal.pone.0044107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KM, Kim YS, Lim JY, Min SJ, Ko HC, Kim SJ, et al. Intestinal anti-inflammatory activity of Sasa quelpaertensis leaf extract by suppressing lipopolysaccharide-stimulated inflammatory mediators in intestinal epithelial Caco-2 cells co-cultured with RAW 264.7 macrophage cells. Nutr Res Pract 2015;9:3–10. 10.4162/nrp.2015.9.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishihara Y, Takemoto T, Itoh K, Ishida A, Yamazaki T. Dual role of superoxide dismutase 2 induced in activated microglia: oxidative stress tolerance and convergence of inflammatory responses. J Biol Chem 2015;290:22805–17. 10.1074/jbc.M115.659151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinarello CA. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am J Clin Nutr 2006;83: 447S–55S. [DOI] [PubMed] [Google Scholar]
- 13.Brenner RR, Rimoldi OJ, Lombardo YB, González MS, Bernasconi AM, Chicco A, et al. Desaturase activities in rat model of insulin resistance induced by a sucrose-rich diet. Lipids 2003;38:733–42. 10.1007/s11745-003-1121-x [DOI] [PubMed] [Google Scholar]
- 14.Shibuya T, Mandai T, Kubota M, Fukuda S, Kurimoto M, Tsujisaka Y. Production of turanose by cyclomaltodextrin glucanotransferase from bacillus stearothermophilus. J Appl Glycosci 2004;51: 223–7. 10.5458/jag.51.223 [DOI] [Google Scholar]
- 15.Wang R, Bae JS, Kim JH, Kim BS, Yoon SH, Park CS, et al. Development of an efficient bioprocess for turanose production by sucrose isomerisation reaction of amylosucrase. Food Chem 2012;132:773–9. 10.1016/j.foodchem.2011.11.035 [DOI] [Google Scholar]
- 16.Pikis A, Immel S, Robrish SA, Thompson J. Metabolism of sucrose and its five isomers by Fusobacterium mortiferum. Microbiology 2002;148:843–52. 10.1099/00221287-148-3-843 [DOI] [PubMed] [Google Scholar]
- 17.Dahlqvist A. Characterization of hog intestinal invertase as a glucosido-invertase. III. Specificity of purified invertase. Acta Chem Scand 1960;14:63–71. 10.3891/acta.chem.scand.14-0063 [DOI] [Google Scholar]
- 18.Park MO, Lee BH, Lim E, Lim JY, Kim Y, Park CS, et al. Enzymatic process for high-yield turanose production and its potential property as an adipogenesis regulator. J Agric Food Chem 2016;64: 4758–64. 10.1021/acs.jafc.5b05849 [DOI] [PubMed] [Google Scholar]
- 19.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods 1986;89:271–7. 10.1016/0022-1759(86)90368-6 [DOI] [PubMed] [Google Scholar]
- 20.Huang Q, Wang T, Wang HY. Ginsenoside Rb2 enhances the anti-inflammatory effect of ω-3 fatty acid in LPS-stimulated RAW264.7 macrophages by upregulating GPR120 expression. Acta Pharmacol Sin 2017;38:192–200. 10.1038/aps.2016.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinkerton JW, Kim RY, Robertson AAB, Hirota JA, Wood LG, Knight DA, et al. Inflammasomes in the lung. Mol Immunol 2017;86:44–55. 10.1016/j.molimm.2017.01.014 [DOI] [PubMed] [Google Scholar]
- 22.Fengyang L, Yunhe F, Bo L, Zhicheng L, Depeng L, Dejie L, et al. Stevioside suppressed inflammatory cytokine secretion by down-regulation of NF-κB and MAPK signaling pathways in LPS-stimulated RAW264.7 cells. Inflammation 2012;35:1669–75. 10.1007/s10753-012-9483-0 [DOI] [PubMed] [Google Scholar]
- 23.Lim E, Lim JY, Kim E, Kim YS, Shin JH, Seok PR, et al. Xylobiose, an alternative sweetener, ameliorates diabetes-related metabolic changes by regulating hepatic lipogenesis and miR-122a/33a in db/db Mice. Nutrients 2016;8:E791. 10.3390/nu8120791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim E, Kim YS, Kim KM, Jung S, Yoo SH, Kim Y. D-Xylose as a sugar complement regulates blood glucose levels by suppressing phosphoenolpyruvate carboxylase (PEPCK) in streptozotocin-nicotinamide-induced diabetic rats and by enhancing glucose uptake in vitro. Nutr Res Pract 2016;10:11–8. 10.4162/nrp.2016.10.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh DK. Tagatose: properties, applications, and biotechnological processes. Appl Microbiol Biotechnol 2007;76:1–8. 10.1007/s00253-007-0981-1 [DOI] [PubMed] [Google Scholar]
- 26.Dye L, Gilsenan MB, Quadt F, Martens VE, Bot A, Lasikiewicz N, et al. Manipulation of glycemic response with isomaltulose in a milk-based drink does not affect cognitive performance in healthy adults. Mol Nutr Food Res 2010;54:506–15. 10.1002/mnfr.200900196 [DOI] [PubMed] [Google Scholar]
- 27.Kawai K, Okuda Y, Yamashita K. Changes in blood glucose and insulin after an oral palatinose administration in normal subjects. Endocrinol Jpn 1985;32:933–6. 10.1507/endocrj1954.32.933 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura K, Ogawa S, Dairiki K, Fukatsu K, Sasaki H, Kaneko T, et al. A new immune-modulating diet enriched with whey-hydrolyzed peptide, fermented milk, and isomaltulose attenuates gut ischemia-reperfusion injury in mice. Clin Nutr 2011;30:513–6. 10.1016/j.clnu.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 29.Hodoniczky J, Morris CA, Rae AL. Oral and intestinal digestion of oligosaccharides as potential sweeteners: a systematic evaluation. Food Chem 2012;132:1951–8. 10.1016/j.foodchem.2011.12.031 [DOI] [Google Scholar]
- 30.MacDonald I, Daniel JW. The bio-availability of isomaltulose in man and rat. Nutr Rep Int 1983;28:1083–90. [Google Scholar]