Figure 5. Production of functional TIMP2 protein by gRNA-induced NSC34 cells.
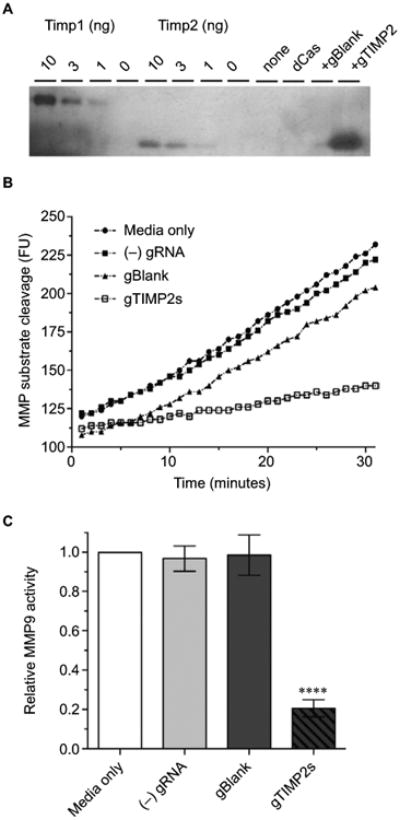
Notes: (A) Reverse zymography of cell culture supernatant The lanes were loaded with control recombinant TIMP1 or 2 (10, 3,1, or 0 ng), or cell culture supernatant from NSC34 cells transfected with nothing, dCas-VPR alone, dCas-VPR with a negative control blank gRNA, or dCas-VPR with all 4 (p-194, p-535p, p-610, and p-956) TIMP2 gRNAs. The transfection condition was identical to that for mRNA assay except that the culture supernatant was harvested 48 hours after transfection. The blot is representative of 3 biological replicates. (B) Time course of increase in fluorescence due to cleavage of the fluorogenic MMP substrate. The slope of the fluorescence vs time plot was taken as a measure of the relative catalytic activity. The plot is representative of 3 biological replicate experiments. (C) A bar plot of relative MMP9 catalytic activity (mean±standard error of the mean, n=3). Note significant inhibition (****P<0.0001) of the catalytic activity by cell culture supernatant from wells transfected with all 4 TIMP2 gRNAs.
Abbreviations: MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases; VPR, VP64-p65-Rta.
