Fig. 7.
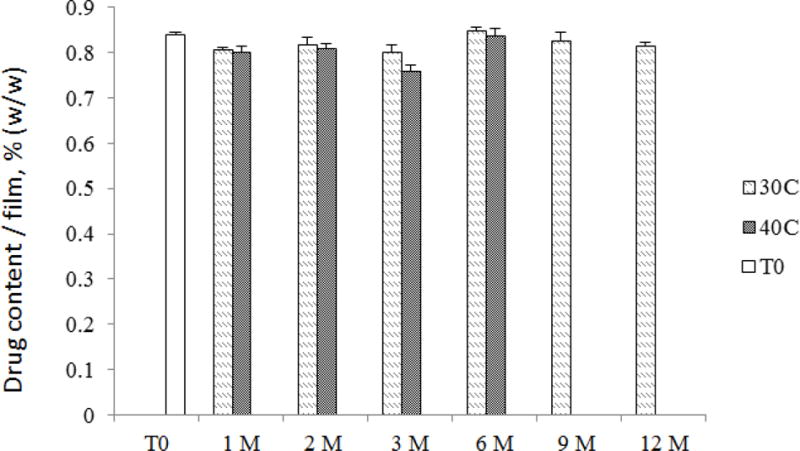
CSIC drug content results from the stability study. CSIC films were stored for 12 months in 30°C/65% RH and 6 months in 40°C/75% RH as per International Council for Harmonisation (ICH) guidelines. Film units were pulled at various time points for evaluation of drug content. The change in drug content was always less than 10% (n=5)
