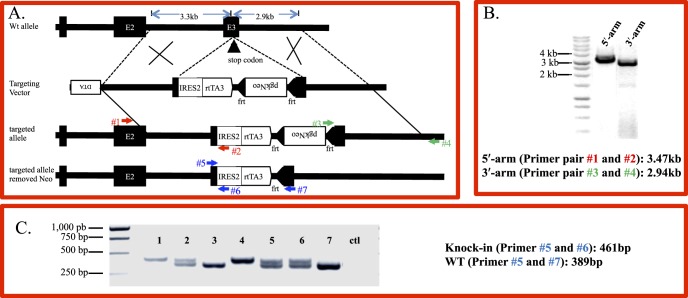
Figure 1.
Generation of keratocyte-specific knock-in mouse line KeraRT via gene targeting. (A) To generate the targeting vector the IRES-rtTA cassette followed by a positive selection marker pgk-Neo minigene was inserted after the Kera exon 3 stop codon. The final targeting vector containing the 3.3 kb 5′-arm, 2.9 kb 3′-arm, and a negative selection marker DTA, was linearized by SacII before being transfected into a C57/BL ES cell line via electroporation. (B) Targeted ES clones were identified by PCR. To identify targeted ES cells, two specific PCR primer pairs detecting the 5′-arm and 3′-arm, respectively, are shown as numbered arrows. Lane 1 is the PCR product using primer #1 and #2 detecting the 5′-arm of targeted clones. Lane 2 is the PCR product using primer #3 and #4 detecting the 3′-arm of targeted clones. (C) Genotyping by PCR of offspring from heterozygous mating. The PCR primer pairs used for identification of the knock-in allele also are shown as numbered arrows. Shown is the expected size of the PCR product amplified by three primers included in one single reaction to distinguish homo-, heterozygous and WT. Lanes 1 and 4 are homozygous knock-in mice. Lane 2, 5, and 6 are heterozygous; while lanes 3 and 7 are WT.