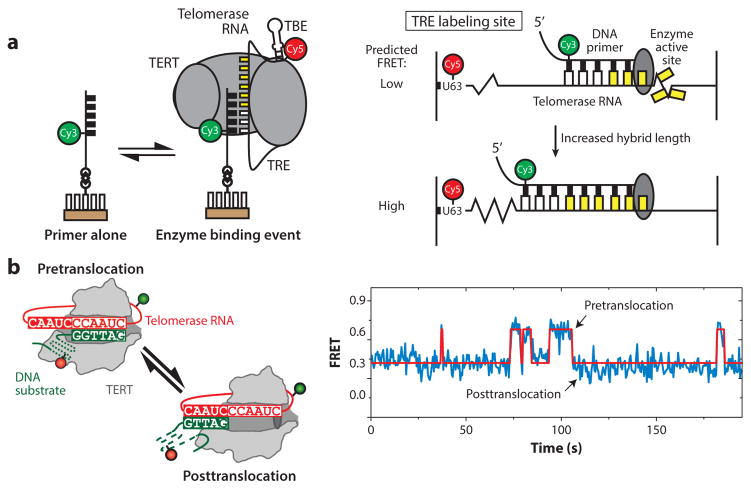
Figure 6.
Intramolecular telomerase dynamics. (a) Single-molecule Förster resonance energy transfer (smFRET) assay measuring internal movement of telomerase RNA throughout the catalytic cycle. RNA sequences flanking the template exhibit reciprocal compression and expansion motion during telomere DNA synthesis. Panel a adapted with permission from Reference 10. (b) DNA substrate dynamics in human telomerase observed by smFRET. Upon completion of one round of telomere repeat synthesis, the 3′ end of the DNA primer exhibits rapid dynamics between the two possible base-pairing registers with the RNA template. Kinetic analysis of these single-molecule data suggests that template hybrid realignment is much faster than the rate-limiting step of telomerase translocation required during repeat addition processivity. Panel b adapted with permission from Reference 84. Abbreviations: TBE, template boundary element; TERT, telomerase reverse transcriptase; TRE, telomeric repeat elongation.