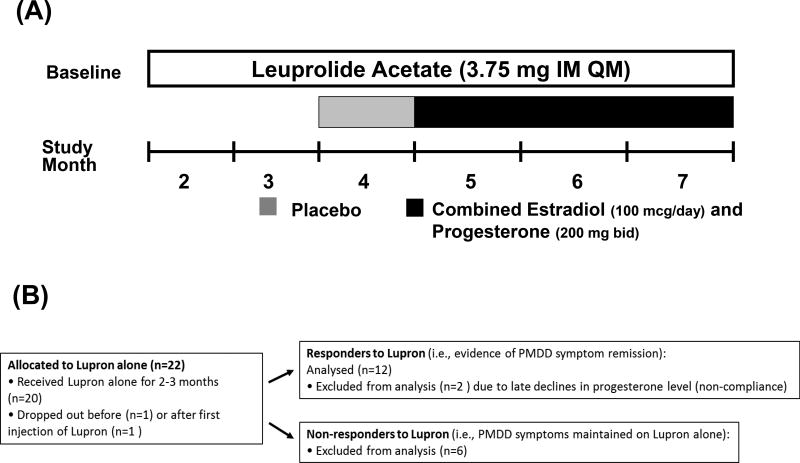
Figure 1.
(A) Study Design Schematic: After a baseline cycle in which the diagnosis of PMDD was established all women received open label leuprolide. Between 2 and 6 days after onset of menses, women received six monthly intramuscular injections of 3.75 mg leuprolide After two - three months of leuprolide alone, those women whose PMDD symptoms were in remission (responders to leuprolide) (i.e., self-reported improvement confirmed by Premenstrual Tension scores <5 and the absence of symptom cyclicity on Daily Rating Form (i.e., weekly [7-day] average daily scores for irritability, sadness or anxiety of < 3 (indicating less than moderate severity of a particular symptom) (52;53) were selected to continue on leuprolide for an additional 4 months. Women not meeting these criteria were considered non-responders and were not included in the statistical analysis. Responders to leuprolide continued to receive monthly leuprolide injections for another four months and received one month of single blind (to participant only) placebo (patch and suppository) followed by three months of combined estradiol (100 ug daily by skin patch) and progesterone (200 mg vaginal suppository twice daily) replacement.
Functional impairment was assessed through self-reports of distress and functional impairment on the Daily Rating Form (23). The Daily Rating Form criteria for functional impairment were as follows: a score of 2 (minimal) or higher on one of 4 questions related to functional impairment (i.e., stayed at home or avoided social activities, had conflicts or problems with people, symptoms interfered with relationships at work or home, or symptoms interfered with work productivity) in at least 3 days out of 7 days pre-menses.
(B) Study Patient Flow Chart