Figure 2. Supercharged sfGFP variants do not irreversibly aggregate at high protein concentration in high trehalose-high histidine buffer.
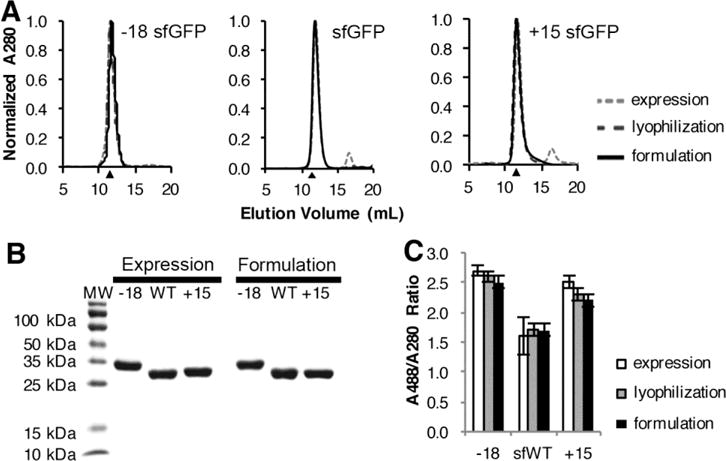
A, Analytical SEC was used to monitor the presence of soluble aggregates at different processing stages using a Superdex 75 column and an ÅKTA FPLC. Chromatograms were collected for a 100 μl injection of 1 mg/ml each sfGFP variant after initial purification, lyophilization, and dilution from high trehalose-high histidine conditions. Protein molecular weight standards indicated that the primary peaks eluted at volumes consistent with the 27–30 kDa sfGFP size; black triangles indicate the expected elution volume of a 27.5 kDa protein based on the calibration curve of protein molecular weight standards (Figure S5). B, SDS-PAGE analysis was used to monitor protein purity and the presence of covalent aggregates. Each sfGFP variant was analyzed after initial purification and after dilution from high concentration. The shift exhibited by the −18 variant is due to the altered electrophoretic mobility of the negatively charge protein. C, The specific activity for each sfGFP variant was measured as the ratio of absorbance at 488 nm divided by the absorbance at 280 nm, which proportional to total protein concentration. Error bars represent the standard deviation of replicate measurements; each experiment was repeated with at least two independent samples.
