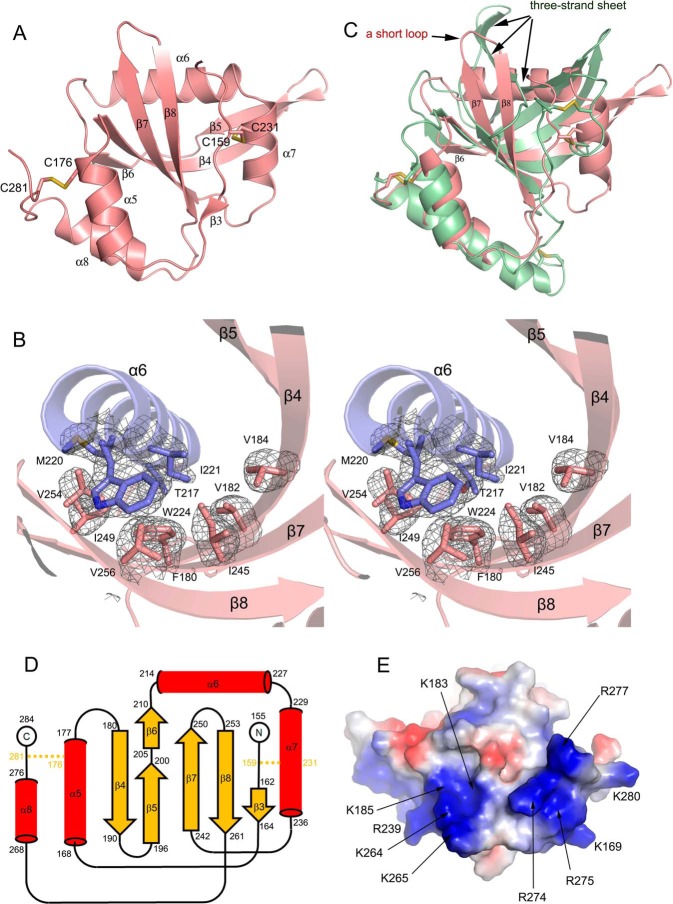
Figure 3.
Structural novelties of Sizzled NTR domain. A, ribbon model of Sizzled NTR domain with disulfide bonds shown in stick representation. B, stereoview of hydrophobic residue contacts among α6 (slate) and the central mixed β-sheet (salmon). The side chains of the residues are shown as sticks. The Fo − Fc electron density map (gray mesh) for these residues were contoured at 3.0 σ. C, overlay of Sizzled NTR (salmon) with human complement C5 NTR domain (grass green). D, secondary-structure diagram of the Sizzled NTR fold. Disulfide bridges are indicated by orange lines. E, bottom surface of Sizzled NTR colored by electrostatic potential from red (negatively charged) to blue (positively charged). Conserved residues are labeled with arrows.