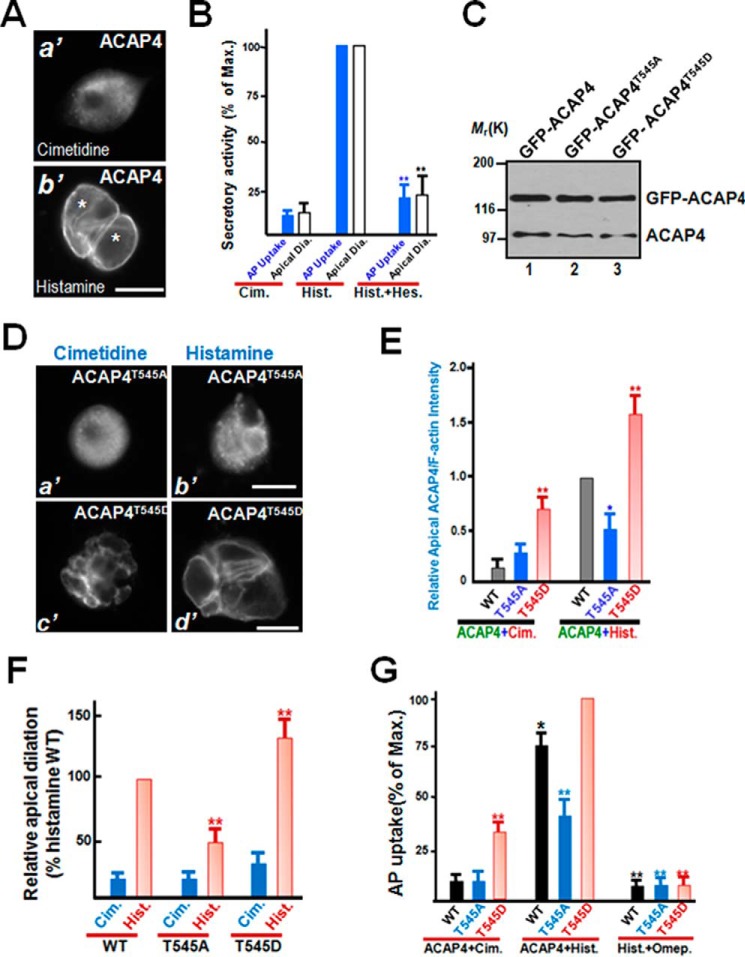
Figure 3.
MST4-elicited phosphorylation of Thr545 is essential for parietal cell activation. A, immunofluorescence staining of ACAP4 in non-secreting and secreting cultured parietal cells. ACAP4 is primarily located to the cytoplasm, with brief concentration at the apical membrane. However, after histamine stimulation, the ACAP4 signal is enriched in dilated apical membrane vacuoles. Scale bar = 10 μm. B, quantitative analyses of apical vacuole diameter as a function of parietal cell secretion. In parallel to morphological quantification of apical vacuole diameter as a function of parietal cell secretion, aliquots of gastric parietal cells were treated with cimetidine (Cim.), histamine (Hist.), and hesperadin (Hes.) plus histamine at 37 °C for 20 min as described under “Experimental procedures,” followed by assessment of secretory activity using AP uptake. **, p < 0.01 compared with histamine-stimulated controls. Max., maximal stimulation. Error bars represent the standard error of three separate preparations in each category. In the case of quantification of apical vacuole diameter (white columns), error bars represent the standard error of three separate preparations of 20 cells in each category. C, characterization of expression of GFP–ACAP4 proteins in gastric parietal cells. Aliquots of parietal cells were transiently transfected to express ACAP4 proteins (wild-type, T545A, and T545D). Twenty-four hours after transfection, cell lysates were prepared and fractionated on SDS-PAGE, followed by Western blot analyses with ACAP4 antibody. Note that the exogenous GFP–ACAP4 proteins expressed 3-fold higher compared with endogenous ACAP4. D, MST4-elicited phosphorylation of ACAP4 at Thr545 orchestrates histamine stimulation. Aliquots of parietal cells were transiently transfected to express ACAP4 proteins (T545A and T545D). 24 h after transfection, the parietal cells were treated with histamine (100 μm) as detailed under “Experimental procedures,” followed by fixation and immunofluorescence analyses. Note that the exogenous ACAP4T545D-expressing cells exhibited larger apical membrane vacuoles even before histamine stimulation. Scale bars = 10 μm. E, quantitative analyses of apical localization of ACAP4-expressing (WT, phospho-mimicking T545D, non-phosphorylatable T545A) parietal cells as a function of activation. *, p < 0.05; **, p < 0.01 compared with those of wild-type ACAP4-expressing cells in secreting and non-secreting status. Error bars represent the standard error of three separate preparations of 20 cells in each category. F, quantitative analyses of apical vacuole diameter of ACAP4-expressing parietal cells as a function of activation. **, p < 0.01 compared with stimulated controls. Error bars represent the standard error of three separate preparations of 50 cells in each category. Typically, the average diameter of apical vacuoles in cimetidine-treated non-secreting parietal cells is 2.9 ± 0.3 μm, whereas the diameter for histamine-stimulated secreting cells is 15.7 ± 1.1 μm. The transfection efficiency is 50–55%, as judged by the GFP signal in transfected preparations under a microscope. G, MST4 phosphorylation of ACAP4 cooperates with PKA activation for optimal parietal cell activation. Aliquots of gastric parietal cells were transiently transfected to express ACAP4 wild-type and phosphomutants, and transfected cells were subjected to assess secretory activity using an AP uptake assay as described under “Experimental procedures.” Aliquots of the proton pump inhibitor omeprazole (100 nm) were included to validate whether histamine-stimulated and ACAP4-dependent acid secretion is mediated by proton pump H,K-ATPase. Note that omeprazole treatment inhibited ACAP4 T545D-dependent acid secretion in response to histamine stimulation. **, p < 0.01; *, p < 0.05 compared with stimulated controls. Error bars represent the standard error of three separate preparations.