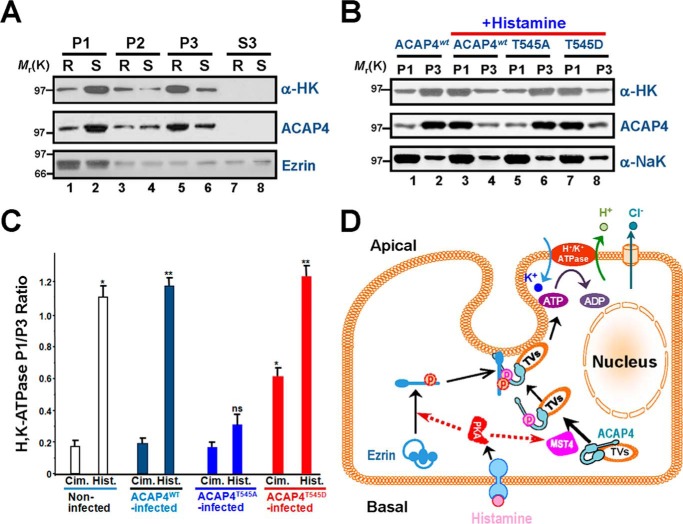
Figure 6.
Phosphorylation of ACAP4 by MST4 elicited relocation of proton pump H,K-ATPase to the apical membrane of parietal cells. A, Western blot analyses of ACAP4, ezrin, and H,K-ATPase (α subunit) of subcellular fractions derived from resting (R, cimetidine) and stimulated (S, histamine) gastric glands. Note that stimulation enriches the protein level of H,K-ATPase (α subunit) in the P1 fraction. B, Western blot analyses of ACAP4, Na,K-ATPase (α subunit), and H,K-ATPase (α subunit) of subcellular fractions derived from resting and stimulated gastric glands infected with GFP–ACAP4 adenovirus (wild-type, ACAP4-T545A, and ACAP4-T545D). C, quantification of the α subunit of H,K-ATPase proteins from P1 (plasma membrane–enriched) and P3 (tubulovesicle-enriched) fractions. The measurements are expressed as the P1/P3 ratio. All data are given as means ± S.E. (error bars) of four preparations. Cim., cimetidine; Hist., histamine. *, p < 0.05; **, p < 0.01. D, working model illustrating how histamine stimulation–elicited PKA–MST4 signaling induces ezrin and ACAP4 conformational change, which enables the association of ACAP4 with ezrin and provides a spatial cue for tubulovesicle translocation. In non-secreting parietal cells, ACAP4 resides on tubulovesicles and relocates apically upon histamine stimulation. Histamine stimulation induces MST4 activation that phosphorylates ACAP4 at Thr545. In addition, phosphorylation of ACAP4 promotes its association with ezrin phosphorylated at Ser66 by PKA (11, 18). Phosphorylation of Ser66 elicits a conformational change of ezrin, which promotes docking and anchoring of ACAP4-containing tubulovesicles at the apical membrane for subsequent fusion of H,K-ATPase–containing vesicles to the apical membrane for the proton pump.