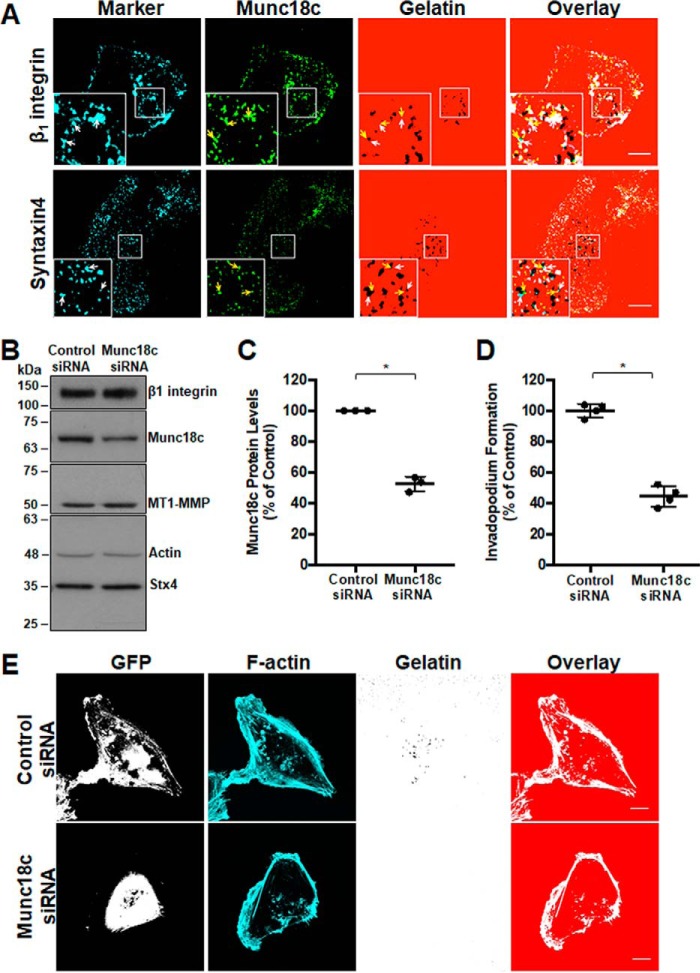
Figure 2.
RNAi-mediated knockdown of Munc18c impairs invadopodium formation. A, distribution of Munc18c during the formation of invadopodia. Cells were analyzed by confocal microscopy after being plated on Alexa Fluor 594–labeled gelatin for 4 h, fixed, permeabilized, and stained as indicated: β1 integrin and Stx4 (cyan, white arrows), Munc18c (green, yellow arrows). Dark spots in the gelatin field indicate sites of gelatin degradation corresponding to invadopodia. B, cells were transfected with siRNA targeting Munc18c or nonspecific control siRNA, lysed, and analyzed for Munc18c by SDS-PAGE/Western blotting. C, quantification of Munc18c knockdown normalized to actin. D, cells with F-actin puncta overlying dark spots of gelatin degradation were counted as cells forming invadopodia. Shown are percentages of cells forming invadopodia, normalized to control (GFP-transfected) cells. E, invadopodium-based degradation of Alexa Fluor 594 gelatin by cells co-transfected with GFP and control siRNA or Munc18c siRNA. Cells were transfected for 44 h, seeded onto gelatin for 4 h, and then fixed, permeabilized, stained for F-actin, and analyzed by confocal microscopy. Percent of controls are from three independent experiments ± S.D. Asterisks denotes values significantly different from control cells (*, p < 0.05). Scale bars = 10 μm. All data represent three or more biological replicates with at least three technical replicates.