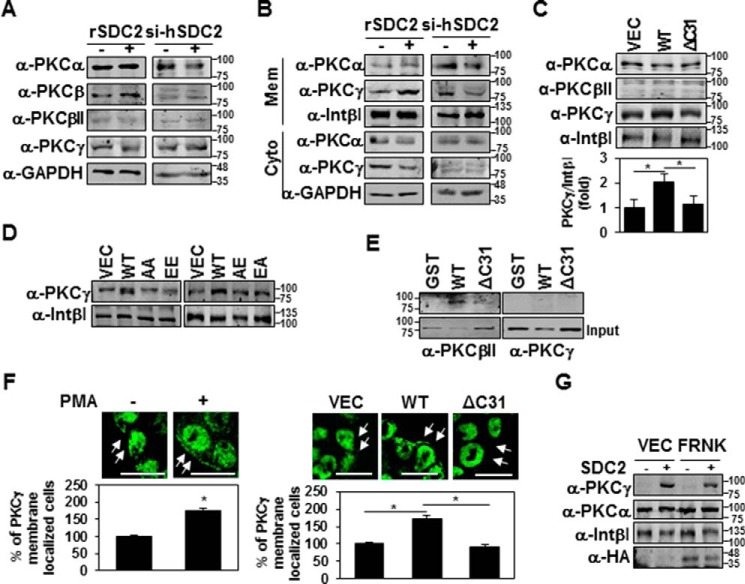
Figure 5.
Syndecan-2 expression increases membrane localization of PKCγ. A and B, HT-29 cells stably transfected with syndecan-2 or si-syndecan-2 (si-hSDC2) oligomer. After 48 h, the PKC isotypes in TCL were determined by immunoblotting with isotype-specific antibodies. GAPDH was used as a loading control (A). The indicated cells were fractionated, and the amounts of the different PKC isotypes in the membrane fraction were analyzed by immunoblotting with isotype-specific PKC antibodies. Integrin β1 was used as a loading control (B). C and D, HT-29 cells stably expressing the syndecan-2, ΔC31, AA, EE, AE, or EA mutant constructs were subjected to membrane fractionation, and the amount of PKCγ in the membrane fraction was analyzed by immunoblotting with an isotype-specific PKC antibody. Integrin β1 was used as a loading control. Data are shown as mean ± S.D. (error bars) (n = 3), normalized to total FAK expression. *, p < 0.05. E, purified GST-SDC2 was incubated with rat brain PKCs for 2 h. Bound proteins were immunoblotted with anti-PKC antibodies. F, HT-29 cells were treated with 20 nm PMA for 4 h and immunostained with the PKCγ antibody. The percentage of PKCγ membrane localization (cells showing membrane localization/total cells) is presented (left). HT-29 cells with or without stable expression of syndecan-2 were immunostained with the PKCγ antibody. The percentage of PKCγ membrane localization (cells showing membrane localization/total cells) is presented (right). Scale bars, 20 μm. Data are shown as mean ± S.D. (n = 3). *, p < 0.05. G, HT-29 cells stably expressing syndecan-2 were transfected with either control (VEC) or an expression vector encoding FRNK. After 24 h, the amount of PKCs in the membrane fraction was analyzed by immunoblotting with an isotype-specific PKC antibody. Integrin β1 was used as a loading control.