Figure 9.
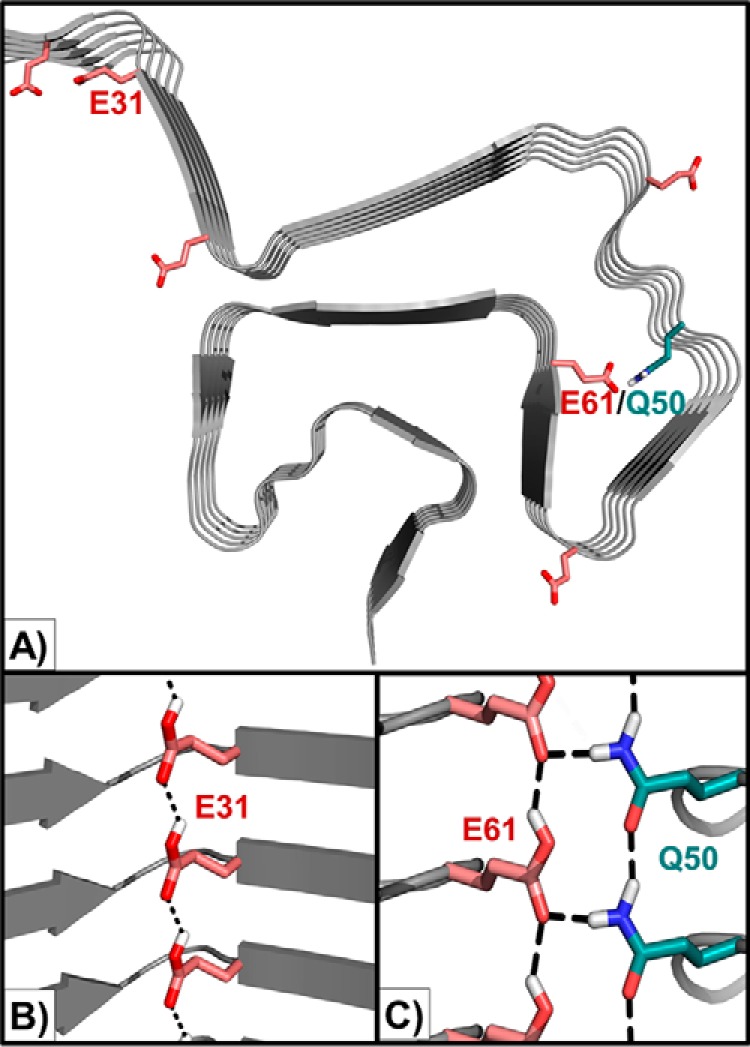
Identified charge-mediated interactions formed by key glutamates in the βS fibril model. A, location of N-terminal glutamate residues (red) and polar side chains in the N-terminal domain in the βS Greek-key fibril model. B, Glu-31 may form a hydrogen-bonded polar zipper ladder in its protonated form (rotameric preferences modeled using glutamine), thereby promoting fibril formation. C, Glu-61 may form a similar ladder and participate in additional interactions with the Gln-50 ladder at low pH. The Gln-50 ladder is predicted to form in a pH-independent manner in the E61A variant.
