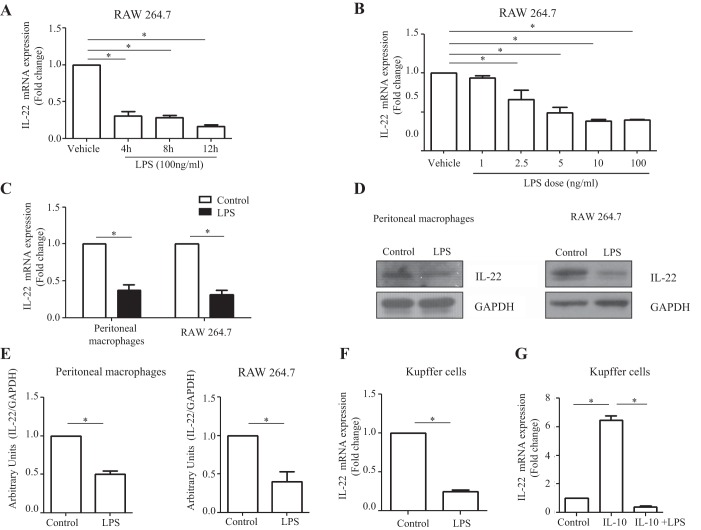
Fig. 2.
Macrophage-derived IL-22 production is downregulated by LPS stimulation. A: RAW 264.7 cells were cultured in 12-well plates overnight and treated with LPS (100 ng/ml) for 4, 8, and 12 h. IL-22 mRNA levels were assayed by RT-qPCR. B: RAW 264.7 cells were seeded in 6-well plates, cultured overnight, and then treated with varying doses of LPS (1, 2.5, 5, 10, and 100 ng/ml) for 8 h. IL-22 mRNA levels were detected by RT-qPCR. C: mouse peritoneal macrophages were isolated and cultured in 12-well plates overnight and then treated with LPS (100 ng/ml) for 10 h. RAW 264.7 cells were seeded in 6-well plates and cultured overnight and then treated with LPS (100 ng/ml) for 10 h. IL-22 mRNA levels were assayed using RT-qPCR. D: IL-22 protein levels were detected in LPS-stimulated macrophages by Western blot. GAPDH was assessed as a leading control. E: densitometric quantification is shown. F: murine Kupffer cells were isolated with a 2-step perfusion-based method and cultured overnight in 24-well plates. Following culture, Kupffer cells were subjected to treatment with LPS (100 ng/ml). LPS treatment significantly reduced IL-22 mRNA expression compared with control cells as quantified by RT-qPCR analysis (n = 3). G: Kupffer cells were stimulated with IL-10 alone or a combination of IL-10 treatment (0.10 ng/ml) followed by LPS (10 ng/ml) treatment. Cells were then cultured for 4 h, and IL-22 mRNA expression was assayed by RT-qPCR. IL-10 treatment significantly enhanced IL-22 mRNA expression; however, LPS stimulation abrogated the effects of IL-10 (n = 3). Values represent means ± SE; *P < 0.05.