Abstract
The carotid body (CB) chemoreflex maintains blood Po2 and Pco2/H+ homeostasis and displays sensory plasticity during exposure to chronic hypoxia. Purinergic signaling via P1 and P2 receptors plays a pivotal role in shaping the afferent discharge at the sensory synapse containing catecholaminergic chemoreceptor (type I) cells, glial-like type II cells, and sensory (petrosal) nerve endings. However, little is known about the family of ectonucleotidases that control synaptic nucleotide levels. Using quantitative PCR (qPCR), we first compared expression levels of ectonucleoside triphosphate diphosphohydrolases (NTPDases1,2,3,5,6) and ecto-5′-nucleotidase (E5′Nt/CD73) mRNAs in juvenile rat CB vs. brain, petrosal ganglia, sympathetic (superior cervical) ganglia, and a sympathoadrenal chromaffin (MAH) cell line. In whole CB extracts, qPCR revealed a high relative expression of surface-located members NTPDase1,2 and E5′Nt/CD73, compared with low NTPDase3 expression. Immunofluorescence staining of CB sections or dissociated CB cultures localized NTPDase2,3 and E5′Nt/CD73 protein to the periphery of type I clusters, and in association with sensory nerve fibers and/or isolated type II cells. Interestingly, in CBs obtained from rats reared under chronic hypobaric hypoxia (~60 kPa, equivalent to 4,300 m) for 5–7 days, in addition to the expected upregulation of tyrosine hydroxylase and VEGF mRNAs, there was a significant upregulation of NTPDase3 and E5′Nt/CD73 mRNA, but a downregulation of NTPDase1 and NTPDase2 relative to normoxic controls. We conclude that NTPDase1,2,3 and E5′Nt/CD73 are the predominant surface-located ectonucleotidases in the rat CB and suggest that their differential regulation during chronic hypoxia may contribute to CB plasticity via control of synaptic ATP, ADP, and adenosine pools.
Keywords: NTPDase1,2,3; ecto-5′-nucleotidase/CD73; hypoxia
in mammals, carotid bodies function as peripheral arterial chemoreceptors that monitor and correct changes in blood O2 and CO2/H+ via initiation of respiratory and cardiovascular reflexes (22, 28). These organs consist of innervated clusters of chemoreceptor (type I) cells in intimate association with sustentacular, glial-like type II cells. Type I cells transduce chemostimuli such as hypoxia (low Po2) and acid hypercapnia (high CO2/H+) and release a variety of excitatory and inhibitory neurochemicals that help shape the afferent discharge carried by the carotid sinus nerve (CSN) (28, 33, 39, 42, 43). The purines ATP and adenosine are among the best studied excitatory neurochemicals that act both postsynaptically and presynaptically to facilitate the CSN discharge during chemoexcitation (2, 17, 28, 40, 42). For example, ATP released from type I cells may act postsynaptically to excite CSN terminals via ionotropic P2X2/3 receptors (45, 49, 63), or presynaptically to stimulate G protein-coupled P2Y2 receptors on adjacent type II cells (53, 58, 62). In addition, adenosine, generated via hydrolysis of extracellular ATP by surface-located enzymes or released though bidirectional equilibrative transporters in type I cells, can further activate presynaptic and postsynaptic P1 receptors (16, 17, 36, 42).
An understanding of the mechanisms regulating carotid body (CB) excitation is of broad interest given that hyperactivity in the CB afferent discharge is associated with several pathophysiological conditions including hypertension, congestive heart failure, obstructive lung disease, diabetes, and sleep apnea (24, 28, 50). The balance between ATP and adenosine levels in the synaptic cleft, determined in part by the affinities and kinetics of the enzymes involved in the catabolism of extracellular ATP, appears a critical component in the control of the CB afferent output. However, little is known about the surface-located enzymes or ectonucleotidases responsible for generating extracellular levels of nucleotides in the CB. Ectonucleotidases catalyze the hydrolysis of tri-, di-, and monophosphate molecules into their respective metabolites which can influence purinergic signaling. For example, degradation of extracellular ATP may help prevent desensitization of postsynaptic P2X receptors at chemosensory synapses (40, 54) or lead to the generation of ligands such as ADP and adenosine, which in turn can produce distinct physiological effects via P2Y and P1 receptors, respectively (19, 60, 65).
Thus far, pharmacological approaches have been used to infer the presence of ectonucleotidases at CB afferent synapses (15, 16, 23, 36); however, their molecular identities remain unknown. There are four large families of ectonucleotidase enzymes: ecto-nucleoside triphosphate diphospho-hydrolase (E-NTPDase/CD39), ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP), alkaline phosphatases, and the ecto-5′-nucleotidase (E5′Nt/CD73). E-NTPDase is the dominant family and its members are known to hydrolyze nucleoside tri- and diphosphate to monophosphate molecules (26). Eight different members of ectonucleotidases (NTPDase1–8) have been identified, of which four (NTPDase1, 2, 3, and 8) are known to be trafficked to and expressed as integral proteins on the cell membrane (4). In the present study, we first used molecular and immunofluorescence approaches to identify and localize the presence of various ectonucleotidases in the rat CB. In addition, we compared expression patterns between the CB and other tissues/cells from the central and peripheral nervous systems. Finally, we tested whether exposure to chronic hypoxia in vivo can regulate ectonucleotidase mRNA expression in rat CB and potentially contribute to CB plasticity during ventilatory acclimatization to hypoxia (28, 44, 56).
MATERIALS AND METHODS
Ethical Approval and Animal Preparation
All animal handling procedures and tissue dissections were performed according to the guidelines of the Canadian Council on Animal Care and approved by McMaster University Animal Research Ethics Board.
Animal Exposure to Hypobaric Hypoxia
Lactating Wistar female rats (Charles River, Saint-Constant, QC, Canada) were housed under controlled 12:12-h light-dark cycle at 22°C and with ad libitum access to food and water. Whole litters of lactating rat pups (postnatal ages P6–7), along with their mothers, were randomly assigned to normoxia (standard laboratory room conditions) or continuous hypobaric hypoxia (60 kPa, simulating an altitude of ~4,300 m). Litters and their mothers were maintained in hypobaric hypoxia using custom-made hypobaric chambers as previously described (35), with initial pressure gradually decreased over an initial period of ~4–5 h and then kept constant at the final pressure for 5–7 days (P11–14). The length of exposure to chronic hypoxia has been previously shown to elicit a significant increase in carotid sinus nerve activity (13). Normobaric (normoxia) groups were kept in standard laboratory conditions. Cages were cleaned, supplemented with adequate bedding, pelleted food and water once, before start of hypoxic exposure, and monitored daily. After chronic hypoxia exposure, rat pups were euthanized by a quick blow to the head that rendered them unconscious and immediately decapitated. Tissues including brain, superior cervical ganglia (SCG), petrosal ganglia (PG), and carotid bodies (CBs), from normoxic and hypoxic groups were harvested and pooled from each litter (representing an experimental replicate, n) in L-15 medium, snap frozen in liquid nitrogen, and stored at −80°C for subsequent experiments.
Cell Cultures
Dissociated carotid body cultures.
Dissociated cell cultures of rat carotid body were prepared as previously described (37, 62, 63). Briefly, juvenile male and female rat pups (~11–12 days old) were rendered unconscious by a blow to the back of the head and immediately decapitated before removal of the carotid bifurcations. Carotid bodies (CBs) were excised and enzymatically dissociated by incubation for 1 h at 37°C in a balanced salt solution containing 0.1% trypsin (Sigma-Aldrich, Oakville, ON, Canada) and 0.1% collagenase (GIBCO, Grand Island, NY). Enzyme solution was replaced with prewarmed modified F-12 medium (GIBCO, Grand Island, NY) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, 1% glutamine, 0.3% glucose, and 3 μg/ml insulin. The CBs were then mechanically teased apart and triturated ~150 times. The cell suspension was plated into the central wells of five to six modified culture dishes; the wells were coated with Matrigel (BD Bioscience, Mississauga, ON, Canada), and the cultures were kept in a humidified incubator at 37°C in 5% CO2-95% air for up to 7 days.
Immortalized chromaffin (MAH) cell cultures.
The v-myc immortalized adrenomedullary chromaffin-derived, HNK1+ (MAH) cell line (6) was grown in modified L-15/CO2 medium (GIBCO) supplemented with 10% fetal bovine serum, 0.6% glucose, 1% penicillin-streptomycin, and 5 μM dexamethasone as previously described (6, 8). For RNA extraction, cells were seeded on 35-mm culture dishes, precoated with poly-d-lysine and laminin, and allowed to grow to subconfluent levels (~75%). Cultures were maintained in a humidified CO2 (5%) incubator, fed every 2–3 days, and passaged every 3–4 days.
Reverse Transcriptase PCR Analysis
Tissues from superior cervical ganglia (SCG), petrosal ganglia (PG), and carotid bodies (CBs) from a litter of 10–12 pups (~11–12 days old) were pooled for total RNA extraction representing an experimental replicate (n). Frozen tissue (brain, SCG, PG, CBs) and MAH cell cultures were grounded in liquid N2 and total RNAs were isolated using Qiagen Kit (Qiagen, Mississauga, ON, Canada) according to manufacturer’s instructions. RNA quality and quantity were determined using a Nanodrop ND1000 (ThermoScientific, Rockford, IL). Aliquots of 5 μl were used to preserve RNA integrity and stored at −80°C. One microgram of DNase-treated RNA/sample was used to generate cDNA using Superscript III and random primers cDNA synthesis kit (Invitrogen, Thermo Fisher, Burlington, ON, Canada) in accordance with manufacturer’s instructions. The absence of genomic DNA contamination was verified by running a sample where no RT was added (−RT). Primers listed below were designed using Primer3 software (http://bioinfo.ut.ee/primer3-0.4.0/) and supplied by MOBIX laboratory (McMaster University, Hamilton, ON, Canada). RT-PCR reactions were carried out using 1 μl of cDNA as a template for PCR reaction in a final volume of 20 μl/reaction. PCR cycling steps were as follows: 95°C for 1 min, 94°C for 1 min, 60°C for 1 min, 72°C for 1 min, and extension at 72°C for 10 min repeated 35 times. Reaction products were analyzed on 1% agarose gel. Primer pairs were used as follows: rat Entpd1, forward 5′-GTCGTCTCACACCAACCTGT-3′, reverse 5′-GCTTTCCATTCTGAGCAAGCG-3′; rat Entpd2, forward 5′-TGATGTTCAAGGTGGTGGCA-3′, reverse 5′-AAAGGGGTACTGTGTGAGCG-3′; rat Entpd3, forward 5′-TGCTTGTGAGCATCGTGGTA-3′, reverse 5′-GTTTGGCTGACCACTCCTGT-3′; rat Entpd5, forward 5′-CCATGTGCCCCGTTAATGTC-3′, reverse 5′-AGCTGTCCTGATGTTCTCTGC-3′; rat Entpd6, forward 5′-TGCCTATATCAAGTGGCACCG-3′, reverse 5′-TGGGAGTTTCTCCAGGTGGC-3′; rat Nt5′e, forward 5′-CTCTCTGTCGGTGGTGAGGT-3′, reverse 5′-CTTTGGAAGGTGGATTTCCTGTG-3′; and rat β-actin, forward 5′-CCT GCA TGC CTC TGG TCG TA-3′, reverse 5′-CCA CT CTT GCT CGA ACT CT-3′. PCR products were sequenced and a blast search using NCBI Blast (http://www.ncbi.nlm.nih.gov) confirmed product specificity.
Quantitative PCR Analysis
Quantitative real-time PCR (qPCR) was performed using CFX96 Touch Real-Time PCR Detection System (Bio-Rad), calibrated for FAM and VIC fluorochromes. Multiplexing reactions were carried out using the TaqMan Universal Master Mix (catalog no. 4440038, Life Technologies, Burlington, ON, Canada) with gene-specific probes conjugated to either FAM or VIC fluorochrome at the 5′-end and the quencher TAMRA at the 3′-end, according to manufacturer’s instructions. Real-time cycling conditions were: 95°C for 10 min, 40 cycles at 95°C for 30 s, and 60°C for 1 min. Potentially stable housekeeping genes were chosen on the basis of previous algorithmic studies showing their relative stability when studying effects of chronic hypoxia on postnatal CB development (25). Housekeeping gene stability was further tested in preliminary experiments where equal amounts of sample cDNA were used. In normoxic and hypoxic samples, TBP was the most stable housekeeping gene (relative to PPIA, LaminA/C, GAPDH, β-actin). Therefore, it was used as the reference gene in data analysis. Data were analyzed using the ΔΔCt method (where ΔCt = CtUnknown − CtReferencegene and ΔΔCt = CtUnknown − CtControl) and are presented as the fold change of mRNA expression in hypoxic relative to normoxic group determined by 2−ΔΔCt method (10). Gene expression assay IDs were as follows: Entpd1, Rn00574887; Entpd2, Rn00596961; Entpd3, Rn00710251; Nt5e, Rn00665212; Lamin A/C, Rn01458766; TBP (TATA-box binding protein), Rn01455646_m1; PPIA (peptidyle-proyl-isomerase A), Rn00690933_m1; GAPDH, Rn01775763_g1.
Double-Label Immunofluorescence
Carotid body sections.
Rat pups (~P11–15) were anesthetized by intraperitoneal administration of pentobarbital sodium (5.5 mg/ml; 0.4 ml), and prewarmed phosphate-buffered saline (PBS) was perfused through the left ventricle followed by fixation with 4% paraformaldehyde in PBS as previously described (62, 63). Briefly, carotid bifurcations were excised and further fixed with 4% paraformaldehyde for 2–3 h at room temperature, before incubation overnight at 4°C in PBS containing 30% sucrose. The bifurcations were cryosectioned (18–20 μm thick) and sections were placed on glass slides coated with 2% silane. Sections were then rehydrated with PBS for 30 min at room temperature and incubated with primary antibodies overnight at 4°C as outlined below.
Cell cultures.
Dissociated rat carotid body cells were grown on modified culture dishes (see above). Cultures were washed with prewarmed PBS and fixed with 4% paraformaldehyde for 30 min at room temperature. After washing with PBS, 3× 3 min each, cultures were incubated with primary antibodies at 4°C.
Antibody staining.
Double-label immunofluorescence procedures were applied to sections and/or cultures as follows. The primary antibodies used were as follows: polyclonal rabbit anti-rat NTPDase2/CD39L1 antibody (rN2-6L; 1:400 dilution), polyclonal rabbit anti-rat NTPDase3/CD39L3 antibody [rN3-1L(I4,I5); rN3-2L(I4,I5); rN3-3L(I4,I5); 1:50 dilution] polyclonal guinea pig anti-rat E5′Nt/CD73 antibody [rNu-4CI4; rNu-5C(I4,I5); rNu-6C(I4,I5);1:400 dilution; Laval University, Quebec, Canada, www.ectonucleotidases-ab.com] (51), monoclonal mouse anti-rat TH antibody (1:2,000; EMD Millipore, Billerica MA), polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:500; Dako, Cedarlane, Burlington, ON, Canada), monoclonal mouse anti-rat neurofilament (68 kDa NF; 1:200 dilution), and monoclonal mouse anti-rat GAP-43 (1:2,000 dilution; Boehringer Mannheim, Montreal, QC, Canada). Primary antibodies were diluted in 1% BSA and 0.5% Triton X-100 in PBS. Cultures and sections were washed with PBS 3× 10 min each followed by incubation with Alexa Fluor 488 green-linked goat anti-rabbit IgG (1:200; Molecular Probes), Alexa Fluor 488 green-linked goat anti-guinea pig IgG (1:75; Thermo Fisher Scientific, Rockford, IL), Alexa Fluor 594 red-linked goat anti-mouse (1:200; Molecular Probes), or Alexa Fluor 594 red-linked goat anti-rabbit (1:200; Molecular Probes) secondary antibodies in 1% BSA and 0.5% Triton X-100 in PBS for 1 h at room temperature in the dark. Sections and cultures were washed 3× 3 min each with PBS and mounted in Vectashield. Fluorescence labeling was visualized using either a Zeiss IM35 inverted microscope equipped with epifluorescence optics, or a Leica TCS SP5 II confocal system, or Zeiss LSM800 laser scanning confocal super resolution detector Airyscan as indicated.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism7 software. Data are expressed as means ± SE and were compared using a parametric unpaired t-test with Welch’s correction. Each individual n value indicates the number of litters used in each experiment. Additionally, for NTPDase2 and NTPDase3 expression in normoxic vs. hypoxic groups, data were also analyzed using a nonparametric (Mann-Whitney) test. In this case, the value for each replicate in both groups was first normalized to one value from the normoxic (control) group before comparison of the two data sets (not shown). Both statistical treatments reached the same conclusion regarding NTPDase2 and NTPDase3 expression in normoxia vs. chronic hypoxia. Asterisks denote significant differences between groups. P < 0.05 was considered statistically significant.
RESULTS
Detection of mRNAs for Various NTPDases and Ecto-5′-Nucleotidase in Rat Carotid Body and in the Central and Peripheral Nervous System
Messenger RNA expression of multiple ectonucleotidases was investigated in extracts of whole rat carotid body (CB), and the expression pattern was compared with other tissues or cells derived from the central and peripheral nervous systems. Specific primers were designed to amplify mRNA expression and products were analyzed on 1% agarose gel. PCR products from the RT-PCR were further confirmed by sequencing and NCBI-BLAST analyses. Using whole brain tissue as a positive control for ectonucleotidase gene expression (66), we examined expression of NTPDase1,2,3,5,6 and E5′Nt/CD73 in the CB, superior cervical ganglion or SCG (example of a peripheral sympathetic ganglion), petrosal ganglion or PG (example of a peripheral sensory ganglion), and in an immortalized adrenal chromaffin cell line (MAH cells) derived from the sympathoadrenal lineage (Fig. 1). The expression pattern of the above ectonucleotidases was variable among these tissues/cells; however, we focused on the established surface-located members NTPDase1,2,3 and E5′Nt, because they were likely to play key roles in CB physiology (see Introduction). Signals for the surface-located members NTPDase1,2,3 and E5′Nt were detected in the CB, though NTPDase5 and 6 mRNAs were also present (Fig. 1). Expression of NTPDase1,2,3 and E5′Nt mRNA was also detectable in the SCG and the PG (Fig. 1), which supplies sensory innervation to the CB. Notably, mRNA expression for NTPDase2,3,5,6 and E5′Nt, but not NTPDase 1, was detectable in chromaffin-derived MAH cells (Fig. 1).
Fig. 1.
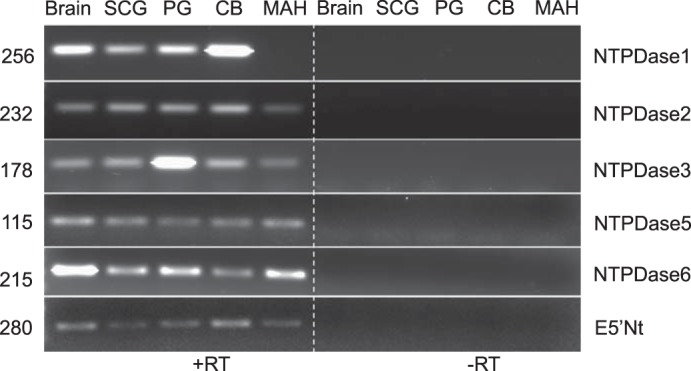
mRNA detection of multiple ectonucleotidase genes in rat carotid body (CB) and tissues/cells from central and peripheral nervous systems. Gel shows RT-PCR products for ectonucleoside triphosphate diphosphohydrolases (NTPDases) 1,2,3,5,6 and ecto-5′-nucleotidase (E5′Nt) obtained from extracts of rat brain, superior cervical ganglion (SCG), petrosal ganglion (PG), whole CB tissue, and the immortalized chromaffin cell line (MAH). No-reverse transcriptase (−RT) samples were used as a negative control (n = 4).
Quantitative Real-Time PCR Analysis of NTPDase1,2,3 and E5′Nt mRNA Expression in the Carotid Body Relative to Brain and Peripheral Ganglia
Using qPCR, we first compared relative expression of NTPDase1,2,3 and E5′Nt mRNA in the CB with that in the brain which expresses all known ectonucleotidases (29). Expression of NTPDase 1,2 and E5′Nt mRNA was significantly higher in CB than in brain (Fig. 2A). By contrast, NTPDase3 expression was significantly lower in CB than in brain. When compared with the SCG, which expresses multiple P2 receptor signaling pathways (11), NTPDase1 and E5′Nt mRNA expression was significantly higher in the CB; no significant difference was observed for NTPDase2 but expression of NTPDase3 was significantly lower in the CB (Fig. 2B). Given the major role of the PG in relaying chemosensory information from the CB to the CNS via purinergic P1 and P2 signaling pathways (17, 40, 42), it was of interest to contrast ectonucleotidase expression between the CB and PG. We found that expression of NTPDase1,2 and E5′Nt mRNA was significantly higher in the CB relative to the PG (Fig. 2C). On the other hand, NTPDase3 expression was significantly lower in the CB relative to the PG. Taken together, these data suggest that NTPDase1,2 and E5′Nt are the dominantly expressed members of the ectonucleotidase family in the CB under normal conditions.
Fig. 2.
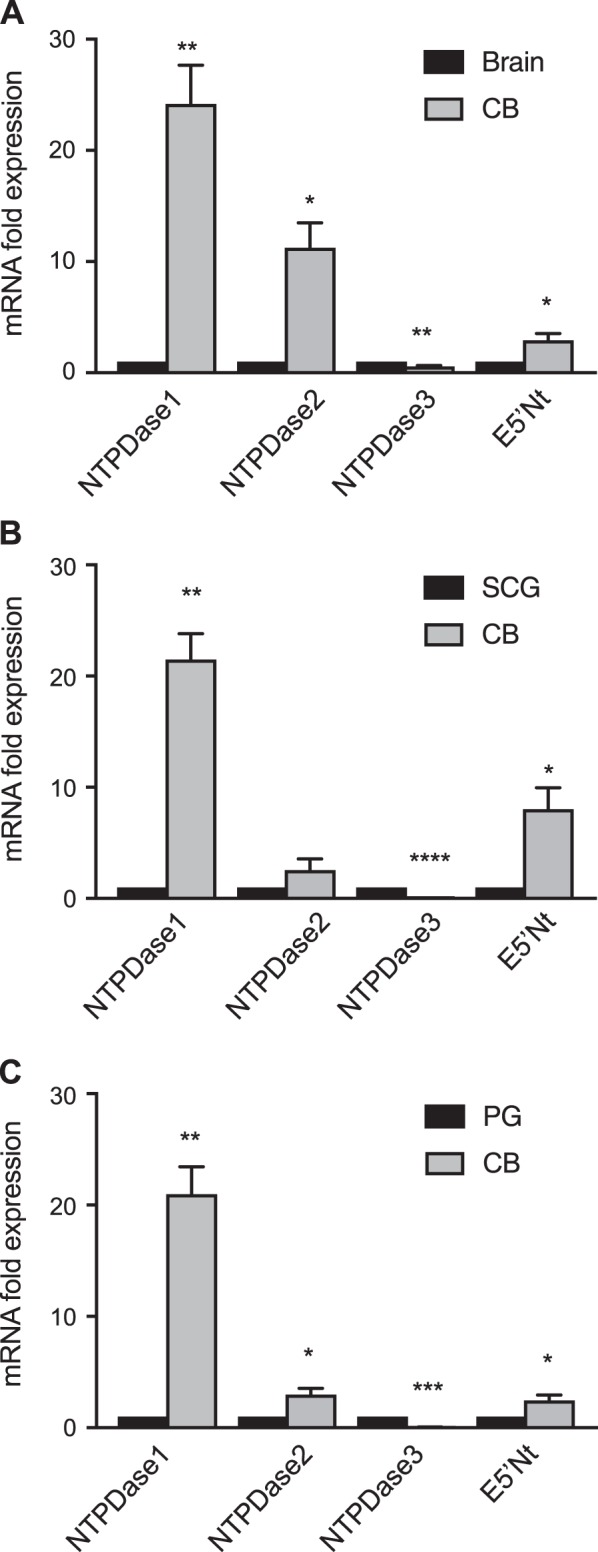
Relative mRNA expression analysis of surface-located ectonucleotidases in rat carotid body (CB). Expression of surface-located ectonucleotidase family members (NTPDase1, 2, 3) and ecto-5′-nucleotidase (E5′Nt) in whole rat CB mRNA extracts were analyzed by quantitative real-time PCR. Data were normalized to housekeeping gene TBP and are shown as fold change relative to NTPDase and E5′Nt mRNA expressed in brain (A), or superior cervical ganglion (SCG) (B), or petrosal ganglion (PG) (C). Each experimental replicate represents expression in tissues pooled from individual rat litters (n). Data are presented as means ± SE for NTPDase expression in CB relative to specified groups; n = 4. Significant differences: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 vs. control tissue, respectively.
Localization of NTPDase2 and NTPDase3 in Tissue Sections of the Rat Carotid Body
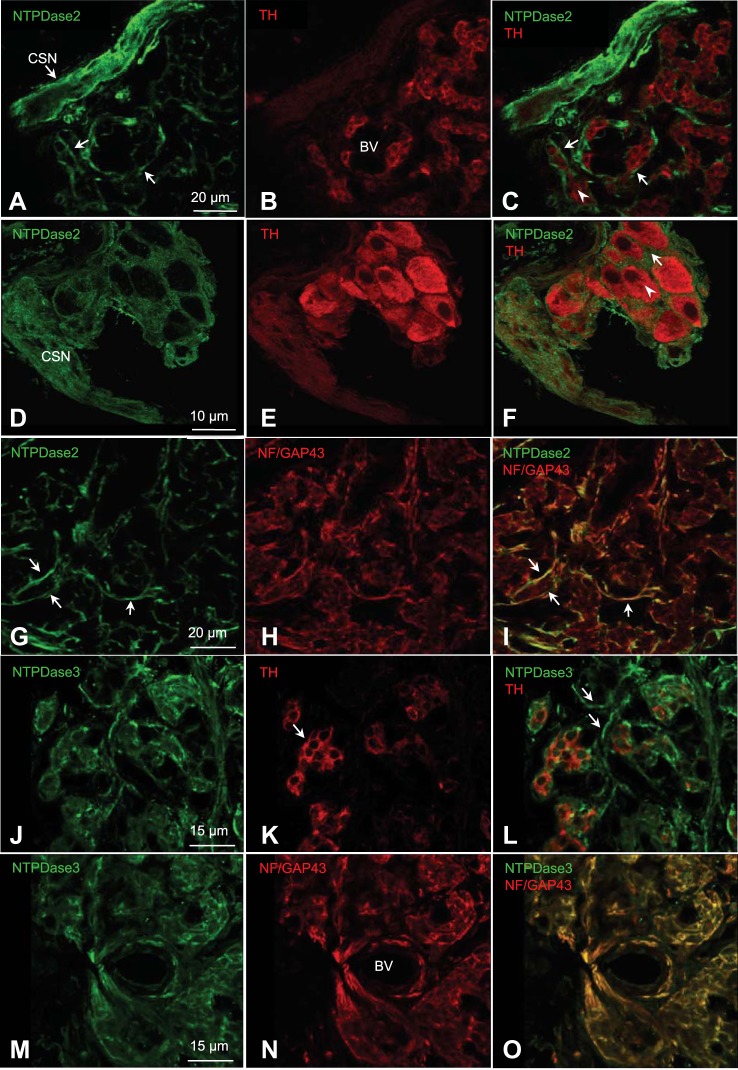
The relatively high expression of NTPDase2 mRNA in the CB, together with the evidence that NTPDase2 is the dominant and functionally important ectonucleotidase in another chemosensory organ, i.e., the taste bud (3, 54), led us to investigate subcellular localization of NTPDase2 in the rat CB using confocal immunofluorescence. In tissue sections of ~2-wk-old rat CB, NTPDase2 immunoreactivity (ir) (green) frequently localized to fibers of carotid sinus nerve (CSN; Fig. 3, A–C), and to the periphery of tyrosine hydroxylase (TH)-positive (red) type I cell clusters (Fig. 3, D–F) where the processes glial-like type II cells are often present (41, 62). Double labeling for both NTPDase2-ir and neurofilament/GAP-43 (NF/GAP-43)-ir confirmed the NTPDase2 staining of nerve fibers in the CB (Fig. 3, G–I). We also tested for the presence of NTPDase3-ir given its known association with sensory nerve fibers (34). Indeed, NTPDase3-ir (green) was localized at the periphery of TH-positive (red) type I cell clusters (Fig. 3, J–L) and in association with NF/GAP-43 positive nerve fibers (Fig. 3, M–O). In control experiments, omission of the primary antibody abolished all immunostaining (data not shown).
Fig. 3.
Confocal immunofluorescence localization of NTPDase2 and NTPDase3 in tissue sections of rat carotid body. A–F: ~2-wk-old carotid body sections, immunostained for NTPDase2 (green; A and D), tyrosine hydroxylase (TH) that labels mainly type I cells (red; B and E), and merged images (C and F). BV, blood vessels. In A and C, the NTPDase2 staining pattern around type I cell clusters may include processes of type II cells. Enlarged images (D–F; arrows) show staining pattern of NTPDase2 surrounding a group of type I cells (C and F; arrowheads). NTPDase2 staining was also associated with the carotid sinus nerve (CSN) as in A and D (n = 4). G–I: colocalization of NTPDase2 (green) and neurofilament/GAP-43 (NF/GAP-43; red) immunoreactivity in nerve processes. J–L: carotid body sections, immunostained for NTPDase3 (green; J), tyrosine hydroxylase (TH) that labels mainly type I cells (red; K), and merged images (L) (n = 3). In J and L, the NTPDase3 staining pattern was associated with type I cell clusters and presumptive nerve processes (arrows). M–O: colocalization of NTPDase3 (green) and neurofilament/GAP-43 (NF/GAP-43; red) immunoreactivity in nerve processes and around blood vessels (BV). Images in A, C, and G–O were acquired using a Leica TCS SP5 II confocal system. D–F were acquired using a Zeiss LSM800 confocal Airyscan system and represent a stack of 7 successive 0.15-μm-thick sections using ImageJ32 (National Institutes of Health, Bethesda, MD).
Localization of NTPDase2, NTPDase3, and E5′Nt in Cell Cultures of Dissociated Rat Carotid Body
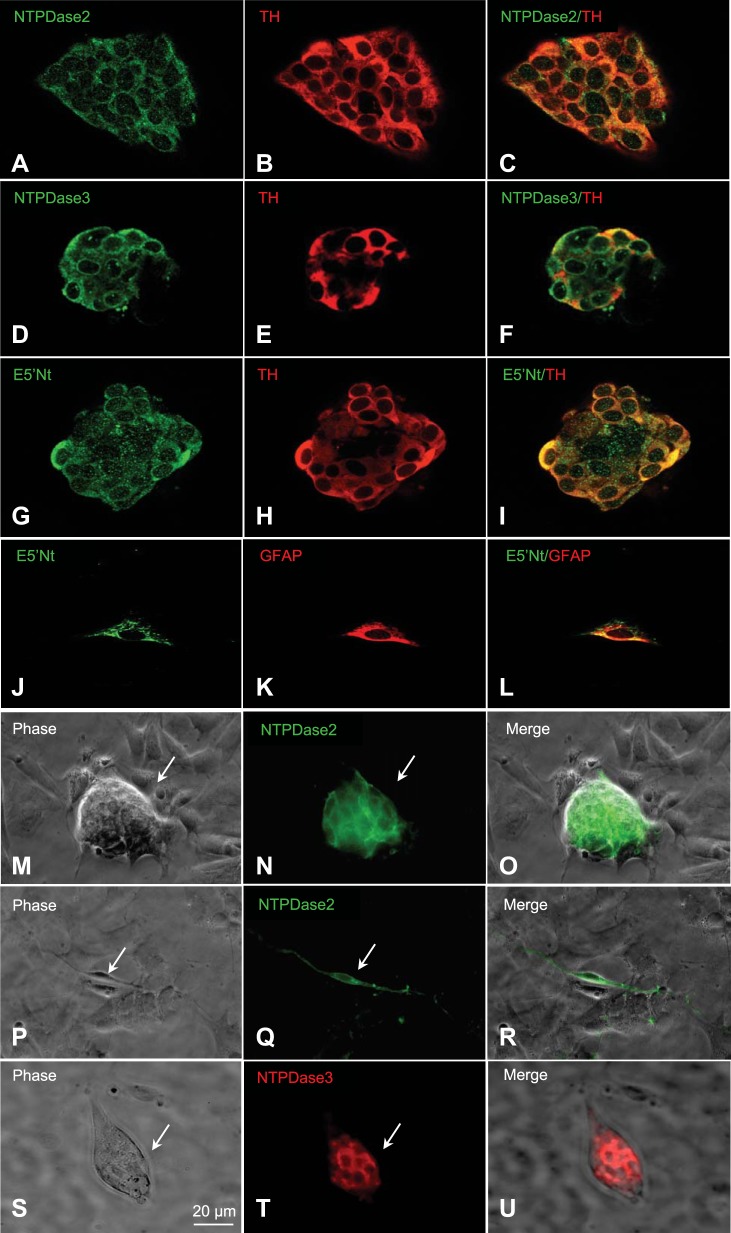
In dissociated rat CB cultures, the presence of functional ecto-5′-nucleotidase (E5′Nt) was recently demonstrated at identifiable chemoreceptor cell clusters using pharmacological tools (36). These clusters typically contain type I cells in intimate association with glial-like type II cells (36, 41, 62). Such cultured preparations allowed us to investigate the localization of NTPDase2,3 and E5′Nt in the absence of contaminating sensory (or autonomic) innervation present in situ. NTPDase2, NTPDase3, and E5′Nt immunostaining (red) was localized to TH-positive (red) type I cell clusters in permeabilized CB culture preparations as illustrated in Fig. 4, A–C, D–F, and G–I, respectively (n = 4). In the case of NTPDase2 and NTPDase3, staining appeared brightest at the periphery of TH-positive type I cells. Occasional isolated glial fibrillary acidic protein (GFAP)-ir type II cells were found to be positively stained for E5′Nt-ir as illustrated in Fig. 4, J–L (n = 3). The extracellular localization of NTPDase2 and NTPDase3 was confirmed in experiments where immunostaining was carried out on nonpermeabilized preparations (n = 3). In these experiments, NTPDase2-ir and NTPDase3-ir were localized specifically to type I clusters as illustrated in Fig. 4, M–O and S–U, respectively; note absence of staining in surrounding background cells in Fig. 4, O and U. The proposal that the glial-like type II cell represents at least one potential site of NTPDase2 localization in the CB was supported in experiments where isolated nonpermeabilized cells with the elongated, spindle-shaped morphology characteristic of type II cells (58, 62), were selectively stained for NTPDase2-ir (Fig. 4, P–R; n = 2).
Fig. 4.
NTPDase2, NTPDase3, and ecto-5′-nucleotidase (E5′Nt) immunoreactivity (ir) in cultures of dissociated rat carotid body (CB). A–F: immunofluorescence staining of permeabilized cultures showing NTPDase2- and NTPDase3-ir (green; A and D, respectively) associated within a TH-ir (red; B and E) type I cell cluster; merged images are shown in C and F (n = 4). G–I: similarly, E5′Nt-ir (green; G) is associated with a TH-ir type I cluster (red; H); merged image is shown in I (n = 3). J–L: demonstration of E5′Nt-ir in an isolated glial fibrillary acidic protein (GFAP)-positive type II cell (n = 2). M–O and S–U: nonpermeabilized CB cultures showing a type I cell cluster (arrow in phase contrast image, M and S), expressing positive, extracellular NTPDase2 and NTPDase3 immunoreactivity (N and T), respectively, and merged images in O and U; note NTPDase2- and -3 negative background cells surrounding the type I cell cluster in N and T (n = 3). P–R: isolated spindle-shaped, putative glial-like type II cell (arrow) in a CB culture is selectively immunopositive for NTPDase2 (green; Q); note NTPDase 2-negative background cells in Q (n = 2).
Regulation of NTPDases and E5′Nt Expression in the Carotid Bodies and Peripheral Ganglia of Rats Exposed to Chronic Hypoxia In Vivo
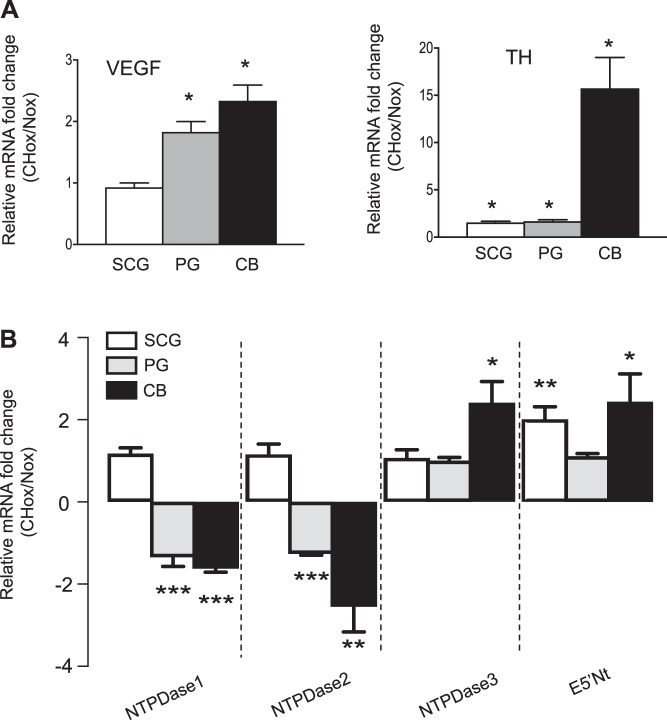
Previous studies demonstrated that chronic hypoxia alters the expression of NTPDase1/CD39 (48, 61) and E5′Nt/CD73 (27) in vascular smooth muscle cells. To learn more about the potential role of hypoxia in regulating ectonucleotidase expression, we exposed juvenile rat pups (P11–14) to chronic hypobaric hypoxia (60 kPa, simulating an altitude of ~4,300 m) for 5–7 days. This duration of hypoxia exposure has been previously shown to significantly increase CB chemosensitivity in adult rats (13). As expected, compared with the control normoxic (Nox) group there was a noticeable increase in CB size in the group exposed to chronic hypoxia (CHox). Measurements of the long and short axes of the ellipsoid CB revealed that the average CB dimensions were 980 ± 114 μm by 750 ± 151 μm for the CHox group (n = 10) vs. 533 ± 58 μm by 467 ± 58 μm for the Nox group (n = 3; P = 0.0001). In addition, there was a significant upregulation of vascular endothelial growth factor (VEGF; Fig. 5A) and tyrosine hydroxylase (TH; Fig. 5B) mRNA expression in CHox compared with Nox CBs. In the PG, both VEGF and TH mRNA were upregulated by chronic hypoxia (Fig. 5A). These data confirm that our experimental protocol correctly predicted the expression pattern for hypoxia-regulated genes in the rat CB (31).
Fig. 5.
Effects of chronic hypoxia (CHox) on NTPDase and ecto-5′-nucleotidase (E5′Nt) mRNA expression in rat carotid body and peripheral ganglia. Approximately 2-wk-old rat pups were exposed to normoxia or hypobaric (60 kPa, 5–7 days) hypoxia. A: quantitative real-time PCR (qPCR) analysis comparing mRNA expression of VEGF and tyrosine hydroxylase (T) in superior cervical ganglion (SCG), petrosal ganglion (PG), and carotid body (CB) tissues isolated from rat pups exposed to chronic hypobaric hypoxia. Data are expressed as mRNA fold change in chronic hypoxia relative to normoxia. B: qPCR analysis of NTPDase1, -2, -3, and E5′Nt mRNA expression in SCG, PG, and CB tissues. Note the significant downregulation of NTPDase1 and NTPDase2 mRNA concomitant with the upregulation of NTPDase3 and E5′Nt in the CB (n = 4 litters). Data were normalized to TBP and are shown as fold change relative to expression in normoxia. Each experimental replicate represents expression in tissues pooled from individual rat litters (n). Significant differences between normoxic and hypoxic groups: *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control, respectively.
Interestingly, qPCR analysis revealed that NTPDase1 and NTPDase2 mRNA were significantly downregulated in the CB and PG from CHox animals compared with Nox controls (Fig. 5B). On the other hand, NTPDase3 and E5′Nt mRNAs were significantly upregulated by ~2.4-fold and ~2.5-fold, respectively, in chronically hypoxic CBs (Fig. 5B). In the PG, neither NTPDase3 nor E5′Nt mRNA was regulated by chronic hypoxia exposure; however in the SCG, only E5′Nt mRNA was significantly upregulated by chronic hypoxia (Fig. 5B). These data suggest that, in the peripheral nervous system, chronic hypoxia differentially regulates expression of surface-located ectonucleotidase in a tissue-specific manner.
DISCUSSION
Purinergic signaling pathways play a central role in regulating the carotid body (CB) afferent discharge during chemoexcitation via both postsynaptic and presynaptic mechanisms involving ionotropic P2X2/3, and G protein-coupled P2Y and P1 receptors (17, 40, 42, 53). In this study, we demonstrate that rat CB expresses multiple surface-located ectonucleotidases, i.e., NTPDase1,2,3 and ecto-5′-nucleotidase (E5′Nt/CD73), which constitute the full cascade for hydrolyzing extracellular ATP to other physiologically active ligands such as ADP and adenosine. Relative to brain and peripheral ganglia (i.e., SCG and petrosal ganglia or PG), NTPDase1,2 and E5′Nt/CD73 mRNA were highly expressed in the CB. NTPDase3 mRNA was also expressed in the CB, though at levels comparable to brain but significantly lower than SCG and PG. Though the cellular localization of NTPDase1 was not determined in the present study, we attribute its high expression mainly to the fact that the CB is reputed to be the most richly vascularized organ in the body (22), and NTPDase1 frequently associates with the vasculature in both sensory (55) and nonsensory tissues (9). Indeed, of all the ectonucleotidases tested, NTPDase1 mRNA was the only one that was not detected in an O2-sensitive sympathoadrenal cell line (MAH cells) that was devoid of blood vessel components. On the other hand, we immunolocalized NTPDase2,3 and E5′Nt to the periphery of CB chemoreceptor clusters in situ and/or in dissociated CB cultures. As discussed further below, those three ectonucleotidases are likely to play important roles in CB synaptic function by regulating purinergic signaling, though we cannot presently exclude a role for NTPDase1 as well. Interestingly, we also found that mRNA expression of the above surface-located ectonucleotidases was differentially regulated in CBs obtained from rats exposed to chronic hypobaric hypoxia (~60 kPa) for 5–7 days. In particular, while there was a significant upregulation of NTPDase3 and E5′Nt/CD73 in chronically hypoxic CBs relative to normoxic ones, there was a downregulation of NTPDase1 and NTPDase2. These data raise the intriguing possibility that differential regulation of surface-located ectonucleotidases, leading to alterations in purinergic P1 and P2 receptor signaling, might contribute to CB plasticity during chronic hypoxia (28, 44, 56).
Localization of NTPDase2,3 and Ecto-5′-Nucleotidase (E5′Nt/CD73) in the Rat Carotid Body
Using immunofluorescence techniques, we localized NTPDase2,3 in tissue sections of the rat CB. In these studies, both NTPDase2 immunoreactivity (NTPDase2-ir) and NTPDase3-ir were prominent around the periphery of chemoreceptor (type I) cell clusters and in association with the carotid sinus nerve (CSN). This CSN association was not unexpected given that, in peripheral sympathetic and sensory ganglia, NTPDase2 is often found associated with satellite glial cells and in nonmyelinating Schwann cells (7), and NTPDase3 has been localized to trigeminal sensory neurons and their axonal projections (34). However, the NTPDase2- and NTPDase3-ir that was localized at the periphery of type I cell clusters appeared to have a nonneural origin as well. In particular, in dissociated CB cultures where all neural elements were absent, NTPDase2- and NTPDase3-ir remained restricted to type I cell clusters. The fact that this staining pattern persisted in nonpermeabilized cultures confirmed an extracellular localization of NTPDase2,3. The observation that occasional isolated cells with the spindle-shape morphology characteristic of type II cells were positive for NTPDase2-ir suggests that type II cells may contribute, at least in part, to the staining pattern observed around type I clusters in CB sections in situ. The NTPDase2 staining pattern is reminiscent of another chemosensory organ, i.e., the mammalian taste bud, where a similar antibody was used to demonstrate NTPDase2 localization to glial-like taste cells (3). Its importance was further confirmed in gene knockout models where NTPDase2 was shown to be the critical ectonucleotidase required for maintaining normal purinergic signaling in the taste bud (3, 54). NTPDase2 was also localized to synaptic regions of sensory inner and outer hair cells of the cochlea (55), suggesting that it may generally play a key role in purinergic signaling among sensory systems. We also confirmed the localization of ecto-5′-nucleotidase (E5′Nt) to type I cell clusters (36), and occasional isolated GFAP-ir type II cells in CB cultures. These data imply that type I cell clusters, which typically contain contiguous type II cells (36, 41), express the complete enzymatic machinery necessary for maintaining purinergic P1 and P2 receptor signaling in the CB.
Regulation of NTPDases and Ecto-5′-Nucleotidase/CD73 Expression by Chronic Hypoxia
Previous studies suggest that exposure to chronic hypoxia can lead to the upregulation of ectonucleotidase expression. For example, chronic hypoxia upregulates NTPDase1/CD39 expression in vascular smooth muscle cells (46–48) and E5′Nt/CD73 in both vascular smooth muscle cells (27) and intestinal epithelia (52). In the latter case, the upregulation of E5′Nt/CD73 was shown to be dependent on the transcription factor hypoxia-inducible factor-1α. Given that CB chemosensitivity is altered following exposure to chronic hypoxia (28, 44), and the importance of purinergic signaling in CB function (see above), it was of interest to investigate ectonucleotidase expression patterns in CBs taken from rats exposed to chronic hypobaric hypoxia for 5–7 days. Surprisingly, relative to normoxic controls, there was a significant downregulation of NTPDase1 and NTPDase2 mRNAs in chronically hypoxic CBs; this contrasted, however, with a significant ~2× upregulation of both NTPDase3 and E5′Nt/CD73 mRNAs. The possible physiological significance of these findings is discussed below. Notably, the upregulation of NTPDase3 mRNA was unique to the CB, and of the remaining tissues (i.e., SCG and PG), only the SCG showed a significant upregulation of E5′Nt/CD73 mRNA in chronically hypoxic animals. The downregulation of NTPDase1 and -2 mRNA was also seen in the PG, but was absent in SCG, suggesting a tissue-specific pattern of ectonucleotidase mRNA regulation during chronic hypoxia.
Possible Physiological Significance of Ectonucleotidase Expression Patterns in Normoxic vs. Hypoxic Carotid Bodies
The specific distribution of ectonucleotidases at the sensory synapse, together with the presence of P1 and P2 receptors on different cell types, ultimately regulates purinergic signaling and therefore CB function. In this study, we show that NTPDase2,3 and ecto-5′-nucleotidase (E5′Nt/CD73) are key surface-located ectonucleotidases expressed at CB chemoreceptors. Their location in the region of the tri-partite synapse formed by chemoreceptor type I cells, glial-like type II cells, and petrosal sensory nerve endings is ideal for regulating ATP and adenosine signaling during chemotransduction (16, 23, 36, 40). It appears that NDPDase2 was selected to play a central role in purinergic signaling in sensory systems given its importance during taste (54) and auditory (55) transduction. NTPDase2 hydrolyses ATP and knockout of the Entpd2 gene in mice is known to cause an increase in extracellular ATP in the taste bud and blunting of taste-evoked responses, attributable to postsynaptic P2X2/3 receptor (P2X2/3R) desensitization (54). Given the importance of postsynaptic P2X2/3R in the CB afferent response to chemostimuli (40), it is likely that NTPDase2 plays a similar role in the CB, i.e., prevention of P2X2/3R desensitization. However, expression of the NTPDase2 alone, in combination with E5′Nt/CD73, is unlikely to generate significant amounts of adenosine because NTPDase2 primarily hydrolyzes ATP to ADP, with minimal effect on ADP hydrolysis (20, 65). Thus, despite its apparently lower expression in normoxic conditions, at least at the mRNA level, synaptic NTPDase3 is likely to play a more important role in ensuring rapid formation of adenosine, given that NTPDase3 efficiently hydrolyses both ATP and ADP (65). Thus, by controlling the rate of formation of AMP, the primary substrate for adenosine synthesis, NTPDase3 would appear to play a key role in CB function. Indeed, this role may increase dramatically in CBs of animals reared under chronic hypoxia, given the ~2-fold upregulation of NTPDase3 mRNA and concomitant ~2-fold downregulation of NTPDase2 (and NTPDase1) mRNA. The rate of adenosine formation should be further enhanced under chronic hypoxia by the nearly 2-fold increase in expression of E5′Nt/CD73 mRNA, given that E5′Nt/CD73 controls the rate limiting step in adenosine synthesis via hydrolysis of AMP (65). Assuming that changes in mRNA expression are correlated with parallel changes in protein expression, these combined data suggest a major shift towards adenosine signaling in the CB during chronic hypoxia.
The above ectonucleotidases are expected to differentially regulate three physiologically active ligands in the CB, i.e., ATP, ADP, and adenosine. The excitatory role of ATP released from type I cells during chemotransduction, and acting via postsynaptic ionotropic P2X2/3 receptors (P2X2/3R) on petrosal terminals, has been well described (39, 40, 42). In addition, paracrine ATP-mediated activation of P2Y2 receptors (P2Y2R) on neighboring type II cells may lead to the further release of ATP via pannexin-1 channels (38, 62). As described above, one fate of this extracellular ATP is the generation of adenosine which in turn could further excite both type I cells and afferent nerve terminals via high-affinity A2aR (16, 17, 36, 42, 53, 57). On the other hand, at sufficiently high concentrations, ATP and ADP (generated via hydrolysis of ATP by NTPDase2) could cause negative feedback inhibition of type I cells via P2Y1R (53, 59) and/or P2Y12R (12, 64). Because ADP is the preferred ligand for both those receptors (30), these inhibitory feedback pathways are likely to be suppressed in conditions of chronic hypoxia where the increased expression of NTPDase3 and E5′Nt/CD73, coupled with the decreased expression of NTPDase2, would favor rapid conversion of ATP and ADP to adenosine. The concomitant rapid removal of ATP and ADP would also favor adenosine formation, because both are known natural physiological inhibitors of E5′Nt/CD73 (30). The proposed shift towards adenosine-A2aR signaling during chronic hypoxia could be further enhanced by tendency of E5′Nt/CD73 and A2aR proteins to colocalize in the nervous system, thereby enabling the efficiency of adenosine-A2aR interactions (1). Prevention of adenosine-mediated overexcitation may however occur under chronic hypoxia should adenosine levels become too high, because type I cells also express low-affinity A2bR, as well as the equilibrative nucleoside transporter ENT (15, 16, 21). Stimulation of A2bR on normoxic and chronically hypoxic type I cells by micromolar levels of adenosine has been shown to facilitate the secretion of dopamine (14, 32), which inhibits CB function via pre- and postsynaptic D2 receptors (5, 14, 21). It is likely that the changes in purinergic signaling described above contribute to ventilatory acclimatization to hypoxia (VAH) when CB chemosensitivity is enhanced (28, 44). In this regard, it is noteworthy that 8-SPT, a nonspecific antagonist of adenosine receptors, attenuated the increase in respiratory frequency evoked by an acute hypoxic challenge in rats reared under chronic hypoxia (12% O2) for 7 days (56). Furthermore, in a related study (18), chronic caffeine, a nonspecific adenosine receptor antagonist, abolished the increased CB sensitivity associated with VAH in adult rats via downregulation of A2b receptors in the CB and upregulation of A2a receptors in the petrosal ganglia.
GRANTS
This work was supported by grants to C. A. Nurse from the Canadian Institutes of Health Research (MOP 142469) and to C. A. Nurse and G. B. McClelland from the Natural Sciences and Engineering Research Council of Canada.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S., C.V., G.B.M., and C.A.N. conceived and designed research; S.S. and C.V. performed experiments; S.S. and C.A.N. analyzed data; S.S., C.V., G.B.M., and C.A.N. interpreted results of experiments; S.S. and C.A.N. prepared figures; S.S. and C.A.N. drafted manuscript; S.S., C.V., G.B.M., and C.A.N. edited and revised manuscript; S.S., G.B.M., and C.A.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Caylieh Robertson and Catherine Ivy for technical assistance in the preparation of the hypobaric chambers.
REFERENCES
- 1.Augusto E, Matos M, Sévigny J, El-Tayeb A, Bynoe MS, Müller CE, Cunha RA, Chen JF. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J Neurosci 33: 11390–11399, 2013. doi: 10.1523/JNEUROSCI.5817-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bairam A, Niane LM, Joseph V. Role of ATP and adenosine on carotid body function during development. Respir Physiol Neurobiol 185: 57–66, 2013. doi: 10.1016/j.resp.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol 497: 1–12, 2006. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belcher SM, Zsarnovszky A, Crawford PA, Hemani H, Spurling L, Kirley TL. Immunolocalization of ecto-nucleoside triphosphate diphosphohydrolase 3 in rat brain: implications for modulation of multiple homeostatic systems including feeding and sleep-wake behaviors. Neuroscience 137: 1331–1346, 2006. doi: 10.1016/j.neuroscience.2005.08.086. [DOI] [PubMed] [Google Scholar]
- 5.Benot AR, López-Barneo J. Feedback Inhibition of Ca2+ currents by dopamine in glomus cells of the carotid body. Eur J Neurosci 2: 809–812, 1990. doi: 10.1111/j.1460-9568.1990.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 6.Birren SJ, Anderson DJ. A v-myc-immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron 4: 189–201, 1990. doi: 10.1016/0896-6273(90)90094-V. [DOI] [PubMed] [Google Scholar]
- 7.Braun N, Sévigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 45: 124–132, 2004. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- 8.Brown ST, Nurse CA. Induction of HIF-2α is dependent on mitochondrial O2 consumption in an O2-sensitive adrenomedullary chromaffin cell line. Am J Physiol Cell Physiol 294: C1305–C1312, 2008. doi: 10.1152/ajpcell.00007.2008. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal 10: 3–50, 2014. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622, 2009. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 11.Calvert JA, Atterbury-Thomas AE, Leon C, Forsythe ID, Gachet C, Evans RJ. Evidence for P2Y1, P2Y2, P2Y6 and atypical UTP-sensitive receptors coupled to rises in intracellular calcium in mouse cultured superior cervical ganglion neurons and glia. Br J Pharmacol 143: 525–532, 2004. doi: 10.1038/sj.bjp.0705959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll JL, Agarwal A, Donnelly DF, Kim I. Purinergic modulation of carotid body glomus cell hypoxia response during postnatal maturation in rats. Adv Exp Med Biol 758: 249–253, 2012. doi: 10.1007/978-94-007-4584-1_34. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002. doi: 10.1152/ajplung.00454.2001. [DOI] [PubMed] [Google Scholar]
- 14.Conde SV, Gonzalez C, Batuca JR, Monteiro EC, Obeso A. An antagonistic interaction between A2B adenosine and D2 dopamine receptors modulates the function of rat carotid body chemoreceptor cells. J Neurochem 107: 1369–1381, 2008. doi: 10.1111/j.1471-4159.2008.05704.x. [DOI] [PubMed] [Google Scholar]
- 15.Conde SV, Monteiro EC. Hypoxia induces adenosine release from the rat carotid body. J Neurochem 89: 1148–1156, 2004. doi: 10.1111/j.1471-4159.2004.02380.x. [DOI] [PubMed] [Google Scholar]
- 16.Conde SV, Monteiro EC, Obeso A, Gonzalez C. Adenosine in peripheral chemoreception: new insights into a historically overlooked molecule–invited article. Adv Exp Med Biol 648: 145–159, 2009. doi: 10.1007/978-90-481-2259-2_17. [DOI] [PubMed] [Google Scholar]
- 17.Conde SV, Monteiro EC, Rigual R, Obeso A, Gonzalez C. Hypoxic intensity: a determinant for the contribution of ATP and adenosine to the genesis of carotid body chemosensory activity. J Appl Physiol (1985) 112: 2002–2010, 2012. doi: 10.1152/japplphysiol.01617.2011. [DOI] [PubMed] [Google Scholar]
- 18.Conde SV, Ribeiro MJ, Obeso A, Rigual R, Monteiro EC, Gonzalez C. Chronic caffeine intake in adult rat inhibits carotid body sensitization produced by chronic sustained hypoxia but maintains intact chemoreflex output. Mol Pharmacol 82: 1056–1065, 2012. doi: 10.1124/mol.112.081216. [DOI] [PubMed] [Google Scholar]
- 19.Cunha RA. Regulation of the ecto-nucleotidase pathway in rat hippocampal nerve terminals. Neurochem Res 26: 979–991, 2001. doi: 10.1023/A:1012392719601. [DOI] [PubMed] [Google Scholar]
- 20.Fausther M, Sheung N, Saiman Y, Bansal MB, Dranoff JA. Activated hepatic stellate cells upregulate transcription of ecto-5′-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am J Physiol Gastrointest Liver Physiol 303: G904–G914, 2012. doi: 10.1152/ajpgi.00015.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gauda EB. Gene expression in peripheral arterial chemoreceptors. Microsc Res Tech 59: 153–167, 2002. doi: 10.1002/jemt.10190. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev 74: 829–898, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Holmes AP, Nunes AR, Cann MJ, Kumar P. Ecto-5′-nucleotidase, adenosine and transmembrane adenylyl cyclase signalling regulate basal carotid body chemoafferent outflow and establish the sensitivity to hypercapnia. Adv Exp Med Biol 860: 279–289, 2015. doi: 10.1007/978-3-319-18440-1_32. [DOI] [PubMed] [Google Scholar]
- 24.Iturriaga R, Del Rio R, Idiaquez J, Somers VK. Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease. Biol Res 49: 13, 2016. doi: 10.1186/s40659-016-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim I, Yang D, Tang X, Carroll JL. Reference gene validation for qPCR in rat carotid body during postnatal development. BMC Res Notes 4: 440, 2011. doi: 10.1186/1756-0500-4-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowles AF. The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal 7: 21–45, 2011. doi: 10.1007/s11302-010-9214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koszalka P, Ozüyaman B, Huo Y, Zernecke A, Flögel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sévigny J, Gear A, Weber AA, Molojavyi A, Ding Z, Weber C, Ley K, Zimmermann H, Gödecke A, Schrader J. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res 95: 814–821, 2004. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 28.Kumar P, Prabhakar NR. Peripheral chemoreceptors: function and plasticity of the carotid body. Compr Physiol 2: 141–219, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langer D, Hammer K, Koszalka P, Schrader J, Robson S, Zimmermann H. Distribution of ectonucleotidases in the rodent brain revisited. Cell Tissue Res 334: 199–217, 2008. doi: 10.1007/s00441-008-0681-x. [DOI] [PubMed] [Google Scholar]
- 30.Lecka J, Rana MS, Sévigny J. Inhibition of vascular ectonucleotidase activities by the pro-drugs ticlopidine and clopidogrel favours platelet aggregation. Br J Pharmacol 161: 1150–1160, 2010. doi: 10.1111/j.1476-5381.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, He L, Stensaas L, Dinger B, Fidone S. Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body. Am J Physiol Lung Cell Mol Physiol 296: L158–L166, 2009. doi: 10.1152/ajplung.90383.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livermore S, Nurse CA. Enhanced adenosine A2b receptor signaling facilitates stimulus-induced catecholamine secretion in chronically hypoxic carotid body type I cells. Am J Physiol Cell Physiol 305: C739–C750, 2013. doi: 10.1152/ajpcell.00137.2013. [DOI] [PubMed] [Google Scholar]
- 33.López-Barneo J, Ortega-Sáenz P, Pardal R, Pascual A, Piruat JI. Carotid body oxygen sensing. Eur Respir J 32: 1386–1398, 2008. doi: 10.1183/09031936.00056408. [DOI] [PubMed] [Google Scholar]
- 34.Ma L, Trinh T, Ren Y, Dirksen RT, Liu X. Neuronal NTPDase3 mediates extracellular ATP degradation in trigeminal nociceptive pathway. PLoS One 11: e0164028, 2016. doi: 10.1371/journal.pone.0164028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClelland GB, Hochachka PW, Weber JM. Carbohydrate utilization during exercise after high-altitude acclimation: a new perspective. Proc Natl Acad Sci USA 95: 10288–10293, 1998. doi: 10.1073/pnas.95.17.10288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murali S, Nurse CA. Purinergic signalling mediates bidirectional crosstalk between chemoreceptor type I and glial-like type II cells of the rat carotid body. J Physiol 594: 391–406, 2016. doi: 10.1113/JP271494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murali S, Zhang M, Nurse CA. Angiotensin II mobilizes intracellular calcium and activates pannexin-1 channels in rat carotid body type II cells via AT1 receptors. J Physiol 592: 4747–4762, 2014. doi: 10.1113/jphysiol.2014.279299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murali S, Zhang M, Nurse CA. Evidence that 5-HT stimulates intracellular Ca2+ signalling and activates pannexin-1 currents in type II cells of the rat carotid body. J Physiol 595: 4261–4277, 2017. doi: 10.1113/JP273473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nurse CA. Neurotransmitter and neuromodulatory mechanisms at peripheral arterial chemoreceptors. Exp Physiol 95: 657–667, 2010. doi: 10.1113/expphysiol.2009.049312. [DOI] [PubMed] [Google Scholar]
- 40.Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol 592: 3419–3426, 2014. doi: 10.1113/jphysiol.2013.269829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nurse CA, Fearon IM. Carotid body chemoreceptors in dissociated cell culture. Microsc Res Tech 59: 249–255, 2002. doi: 10.1002/jemt.10199. [DOI] [PubMed] [Google Scholar]
- 42.Nurse CA, Piskuric NA. Signal processing at mammalian carotid body chemoreceptors. Semin Cell Dev Biol 24: 22–30, 2013. doi: 10.1016/j.semcdb.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Peers C, Buckler KJ. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol 144: 1–9, 1995. doi: 10.1007/BF00238411. [DOI] [PubMed] [Google Scholar]
- 44.Powell FL. The influence of chronic hypoxia upon chemoreception. Respir Physiol Neurobiol 157: 154–161, 2007. doi: 10.1016/j.resp.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prasad M, Fearon IM, Zhang M, Laing M, Vollmer C, Nurse CA. Expression of P2X2 and P2X3 receptor subunits in rat carotid body afferent neurones: role in chemosensory signalling. J Physiol 537: 667–677, 2001. doi: 10.1113/jphysiol.2001.012836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robson SC, Marcus AJ, Broekman MJ, Drosopoulos JHF. Thromboregulation by endothelial cells: significance for occlusive vascular diseases. Arterioscler Thromb Vasc Biol 21: 1251–1252, 2001. doi: 10.1161/hq0701.092133. [DOI] [PubMed] [Google Scholar]
- 47.Robson SC, Cooper DK, d’Apice AJ. Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 7: 166–176, 2000. doi: 10.1034/j.1399-3089.2000.00067.x. [DOI] [PubMed] [Google Scholar]
- 48.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost 31: 217–233, 2005. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 49.Rong W, Gourine AV, Cockayne DA, Xiang Z, Ford AP, Spyer KM, Burnstock G. Pivotal role of nucleotide P2X2 receptor subunit of the ATP-gated ion channel mediating ventilatory responses to hypoxia. J Neurosci 23: 11315–11321, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schultz HD, Marcus NJ, Del Rio R. Role of the carotid body chemoreflex in the pathophysiology of heart failure: a perspective from animal studies. Adv Exp Med Biol 860: 167–185, 2015. doi: 10.1007/978-3-319-18440-1_19. [DOI] [PubMed] [Google Scholar]
- 51.Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, Imai M, Zimmermann H, Robson SC. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood 99: 2801–2809, 2002. doi: 10.1182/blood.V99.8.2801. [DOI] [PubMed] [Google Scholar]
- 52.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest 110: 993–1002, 2002. doi: 10.1172/JCI0215337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tse A, Yan L, Lee AK, Tse FW. Autocrine and paracrine actions of ATP in rat carotid body. Can J Physiol Pharmacol 90: 705–711, 2012. doi: 10.1139/y2012-054. [DOI] [PubMed] [Google Scholar]
- 54.Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci USA 110: 14789–14794, 2013. doi: 10.1073/pnas.1309468110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlajkovic SM, Thorne PR, Sévigny J, Robson SC, Housley GD. NTPDase1 and NTPDase2 immunolocalization in mouse cochlea: implications for regulation of p2 receptor signaling. J Histochem Cytochem 50: 1435–1441, 2002. doi: 10.1177/002215540205001102. [DOI] [PubMed] [Google Scholar]
- 56.Walsh MP, Marshall JM. The early effects of chronic hypoxia on the cardiovascular system in the rat: role of nitric oxide. J Physiol 575: 263–275, 2006. doi: 10.1113/jphysiol.2006.108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu F, Xu J, Tse FW, Tse A. Adenosine stimulates depolarization and rise in cytoplasmic [Ca2+] in type I cells of rat carotid bodies. Am J Physiol Cell Physiol 290: C1592–C1598, 2006. doi: 10.1152/ajpcell.00546.2005. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Tse FW, Tse A. ATP triggers intracellular Ca2+ release in type II cells of the rat carotid body. J Physiol 549: 739–747, 2003. doi: 10.1113/jphysiol.2003.039735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu J, Xu F, Tse FW, Tse A. ATP inhibits the hypoxia response in type I cells of rat carotid bodies. J Neurochem 92: 1419–1430, 2005. doi: 10.1111/j.1471-4159.2004.02978.x. [DOI] [PubMed] [Google Scholar]
- 60.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783: 673–694, 2008. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 61.Yegutkin GG, Helenius M, Kaczmarek E, Burns N, Jalkanen S, Stenmark K, Gerasimovskaya EV. Chronic hypoxia impairs extracellular nucleotide metabolism and barrier function in pulmonary artery vasa vasorum endothelial cells. Angiogenesis 14: 503–513, 2011. doi: 10.1007/s10456-011-9234-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Piskuric NA, Vollmer C, Nurse CA. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. J Physiol 590: 4335–4350, 2012. doi: 10.1113/jphysiol.2012.236265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang M, Zhong H, Vollmer C, Nurse CA. Co-release of ATP and ACh mediates hypoxic signalling at rat carotid body chemoreceptors. J Physiol 525: 143–158, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou T, Chien MS, Kaleem S, Matsunami H. Single cell transcriptome analysis of mouse carotid body glomus cells. J Physiol 594: 4225–4251, 2016. doi: 10.1113/JP271936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362: 299–309, 2000. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 66.Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8: 437–502, 2012. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]