Fig. 7.
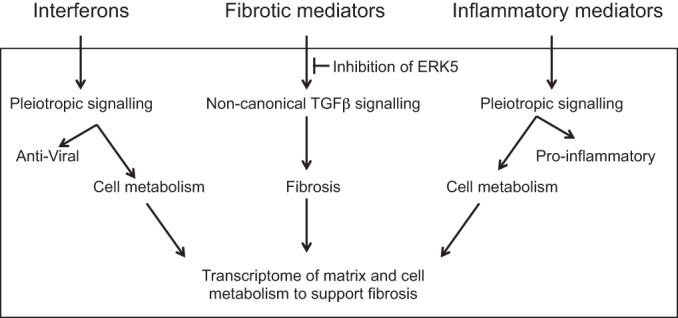
Interferogenic, profibrotic and inflammatory genes and their impact on the phenotype of fibroblast toward fibrosis pathways of autoimmune CHB. Upstream pathological events result in the release of IFNs, fibrotic, and inflammatory mediators by human macrophages treated with immune complexes of Ro60-hY3 and anti-Ro60. The highly reactive fibroblast transcriptome reflects an hY3 macrophage supernatant-enriched environment. For IFNs and inflammatory mediators in addition to well-documented antiviral and pro-inflammatory actions, it has been shown that type I IFN and inflammatory C3A promotes the expression of key component-controlling metabolic gene expression, which, when upregulated, may contribute to the cellular metabolism that is needed to support fibrosis. While “canonical pathways” represent common properties related to the SMAD3 pathway, noncanonical pathways, such as MAPKs, inclusive of MEK5/ERK5, are those that deviated from the canonical paradigm. In our model, for the association of phenotype and fibroblasts treated with hY3 macrophage supernatants, transforming growth factor (TGF)-β signaling by noncanonical is supportive. Specific inhibition of ERK5 with the small molecule BIX-02189 may forestall fibrosis.
